Clin Exp Otorhinolaryngol.
2012 Sep;5(3):117-121. 10.3342/ceo.2012.5.3.117.
Decreased Immunoreactivities of the Chloride Transporters, KCC2 and NKCC1, in the Lateral Superior Olive Neurons of Kanamycin-treated Rats
- Affiliations
-
- 1Department of Otorhinolaryngology-Head & Neck Surgery, Dankook University College of Medicine, Cheonan, Korea.
- 2Department of Physiology, Dankook University College of Medicine, Cheonan, Korea. ansil67@hanmail.net
- KMID: 2134593
- DOI: http://doi.org/10.3342/ceo.2012.5.3.117
Abstract
OBJECTIVES
From our previous study about the weak expressions of potassium-chloride (KCC2) and sodium-potassium-2 chloride (NKCC1) co-transporters in the lateral superior olive (LSO) in circling mice, we hypothesized that partially damaged cochlea of circling mice might be a cause of the weak expressions of KCC2 or NKCC1. To test this possibility, we reproduced the altered expressions of KCC2 and NKCC1 in the LSO of rats, whose cochleae were partially destroyed with kanamycin.
METHODS
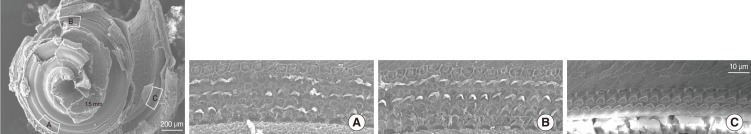
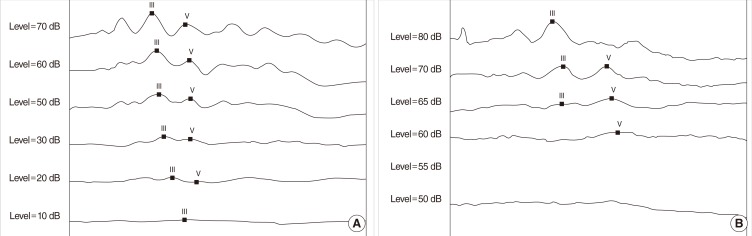
Rat pups were treated with kanamycin from postnatal (P)3 to P8 (700 mg/kg, subcutaneous injection, twice a day) and sacrificed for immunohistochemical analysis, scanning electron microscope (SEM) and auditory brain stem response.
RESULTS
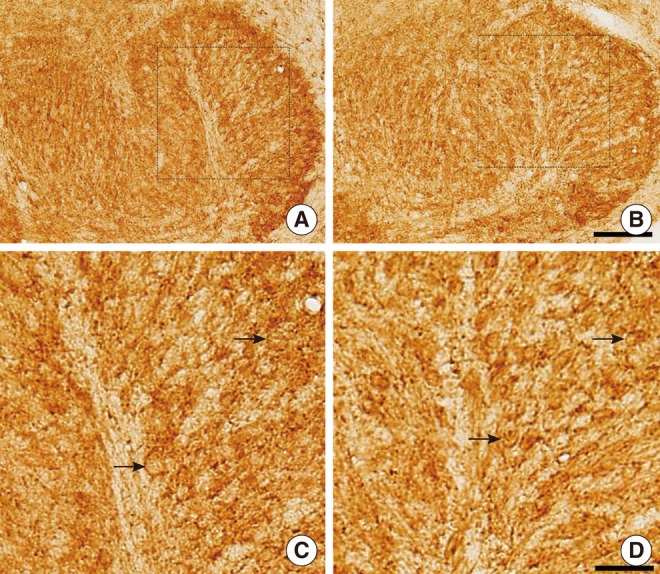
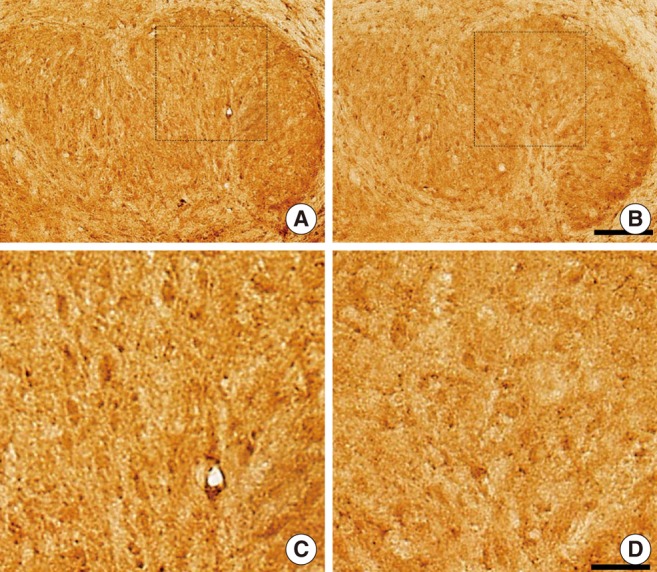
The SEM study revealed partially missing hair cells in P9 rats treated with kanamycin, and the hearing threshold was elevated to 63.8+/-2.5 dB SPL (4 ears) at P16. Both KCC2 and NKCC1 immunoreactivities were more prominent in control rats on P16. On 9 paired slices, the mean densities of NKCC1 immunoreactivities were 118.0+/-1.0 (control) and 112.2+/-1.2 (kanamycin treated), whereas those of KCC2 were 115.7+/-1.5 (control) and 112.0+/-0.8 (kanamycin treated).
CONCLUSION
We concluded that weak expressions of KCC2 and NKCC1 in circling mice were due to partial destruction of cochleae.
Keyword
MeSH Terms
Figure
Reference
-
1. Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci. 1995; 10. 15(10):6890–6904. PMID: 7472446.
Article2. Lohrke S, Srinivasan G, Oberhofer M, Doncheva E, Friauf E. Shift from depolarizing to hyperpolarizing glycine action occurs at different perinatal ages in superior olivary complex nuclei. Eur J Neurosci. 2005; 12. 22(11):2708–2722. PMID: 16324105.3. Cherubini E, Rovira C, Gaiarsa JL, Corradetti R, Ben Ari Y. GABA mediated excitation in immature rat CA3 hippocampal neurons. Int J Dev Neurosci. 1990; 8(4):481–490. PMID: 2174638.
Article4. Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol. 1996; 7. 494(Pt 2):451–464. PMID: 8842004.
Article5. Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991; 2. 65(2):247–263. PMID: 1673153.
Article6. Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J Neurophysiol. 1998; 11. 80(5):2608–2620. PMID: 9819267.
Article7. Wu WL, Ziskind-Conhaim L, Sweet MA. Early development of glycine- and GABA-mediated synapses in rat spinal cord. J Neurosci. 1992; 10. 12(10):3935–3945. PMID: 1403091.
Article8. Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997; 11. 33(6):781–795. PMID: 9369151.
Article9. Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, et al. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999; 1. 397(6716):251–255. PMID: 9930699.
Article10. Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl- uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol. 2004; 6. 557(Pt 3):829–841. PMID: 15090604.11. Pradhan J, Maskey D, Park KS, Kim MJ, Ahn SC. Decreased immunoreactivities and functions of the chloride transporters, KCC2 and NKCC1, in the lateral superior olive neurons of circling mice. Clin Exp Otorhinolaryngol. 2011; 3. 4(1):18–23. PMID: 21461058.
Article12. Chung WH, Kim KR, Cho YS, Cho DY, Woo JH, Ryoo ZY, et al. Cochlear pathology of the circling mouse: a new mouse model of DFNB6. Acta Otolaryngol. 2007; 3. 127(3):244–251. PMID: 17364360.
Article13. Lee JW, Lee EJ, Hong SH, Chung WH, Lee HT, Lee TW, et al. Circling mouse: possible animal model for deafness. Comp Med. 2001; 12. 51(6):550–554. PMID: 11924819.14. Lee JW, Ryoo ZY, Lee EJ, Hong SH, Chung WH, Lee HT, et al. Circling mouse, a spontaneous mutant in the inner ear. Exp Anim. 2002; 4. 51(2):167–171. PMID: 12012726.
Article15. Carlier E, Pujol R. Supra-normal sensitivity to ototoxic antibiotic of the developing rat cochlea. Arch Otorhinolaryngol. 1980; 226(3):129–133. PMID: 7458747.
Article16. Marot M, Uziel A, Romand R. Ototoxicity of kanamycin in developing rats: relationship with the onset of the auditory function. Hear Res. 1980; 3. 2(2):111–113. PMID: 7364666.
Article17. Onejeme AU, Khan KM. Morphologic study of effects of kanamycin on the developing cochlea of the rat. Teratology. 1984; 2. 29(1):57–71. PMID: 6701807.
Article18. Osako S, Tokimoto T, Matsuura S. Effects of kanamycin on the auditory evoked responses during postnatal development of the hearing of the rat. Acta Otolaryngol. 1979; 88(5-6):359–368. PMID: 532611.
Article19. Lee JH, Pradhan J, Maskey D, Park KS, Hong SH, Suh MW, et al. Glutamate co-transmission from developing medial nucleus of the trapezoid body: lateral superior olive synapses is cochlear dependent in kanamycin-treated rats. Biochem Biophys Res Commun. 2011; 2. 405(2):162–167. PMID: 21215254.20. Hong SH, Kim MJ, Ahn SC. Glutamatergic transmission is sustained at a later period of development of medial nucleus of the trapezoid body-lateral superior olive synapses in circling mice. J Neurosci. 2008; 11. 28(48):13003–13007. PMID: 19036993.
Article21. Balakrishnan V, Becker M, Lohrke S, Nothwang HG, Guresir E, Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J Neurosci. 2003; 5. 23(10):4134–4145. PMID: 12764101.
Article22. Shibata S, Kakazu Y, Okabe A, Fukuda A, Nabekura J. Experience-dependent changes in intracellular Cl- regulation in developing auditory neurons. Neurosci Res. 2004; 2. 48(2):211–220. PMID: 14741396.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Decreased Immunoreactivities and Functions of the Chloride Transporters, KCC2 and NKCC1, in the Lateral Superior Olive Neurons of Circling Mice
- Melatonin modulates nitric oxide-regulated WNK-SPAK/OSR1-NKCC1 signaling in dorsal raphe nucleus of rats
- Ca2+ is a Regulator of the WNK/OSR1/NKCC Pathway in a Human Salivary Gland Cell Line
- Effects of kainic Acid-induced seizures on GABA and GABA transporter in the cerebellum of the rat
- Loss of MicroRNA-137 Impairs the Homeostasis of Potassium in Neurons via KCC2