Korean J Urol.
2015 Mar;56(3):187-196. 10.4111/kju.2015.56.3.187.
Structural modifications of the prostate in hypoxia, oxidative stress, and chronic ischemia
- Affiliations
-
- 1Department of Urology, VA Boston Healthcare System and Boston University School of Medicine, Boston, MA, USA.
- 2Department of Urology and Department of Pathology, VA Boston Healthcare System and Boston University School of Medicine, Boston, MA, USA. kazadzoi@bu.edu
- KMID: 2133286
- DOI: http://doi.org/10.4111/kju.2015.56.3.187
Abstract
- PURPOSE
Clinical studies have reported a correlation between pelvic ischemia and voiding dysfunction in elderly men. The aim of this study was to identify and compare prostate structural modifications in cultured cells and in a rabbit model after exposure to hypoxia, oxidative stress, and chronic ischemia.
MATERIALS AND METHODS
Cultured human prostate smooth muscle cells (SMCs), epithelial cells (ECs), and stromal cells (SCs) were incubated under normoxia, hypoxia, and oxidative stress conditions by use of a computerized oxycycler system. We developed a rabbit model of chronic prostate ischemia by creating aorto-iliac arterial atherosclerosis. Markers of oxidative stress were examined by using fluorometric analysis and enzyme immunoassay. Prostate structure was examined by using Masson's trichrome staining and transmission electron microscopy (TEM).
RESULTS
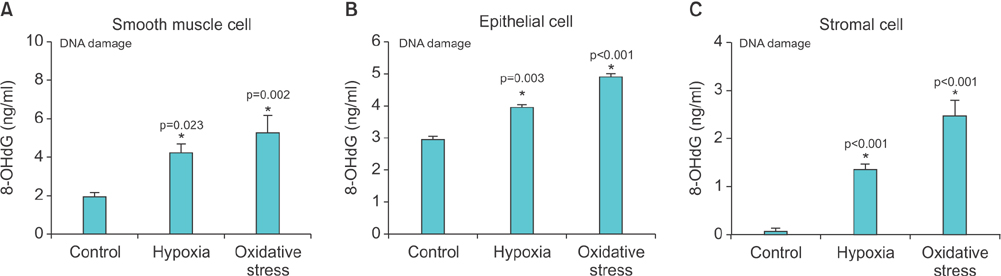
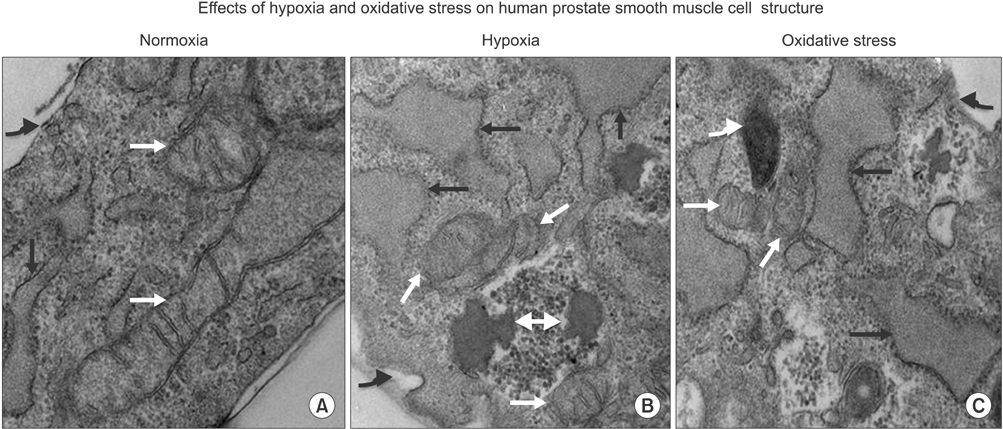
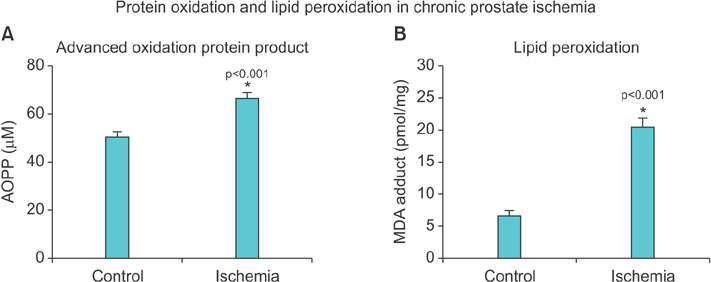
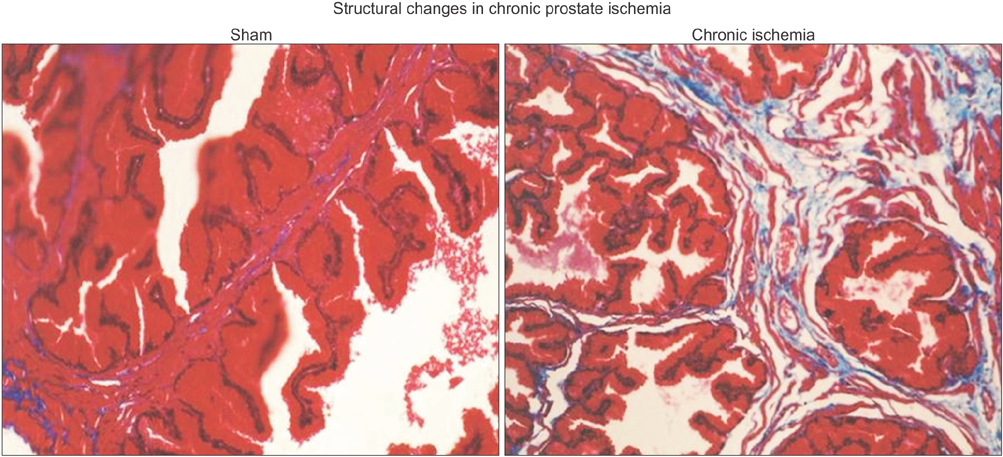
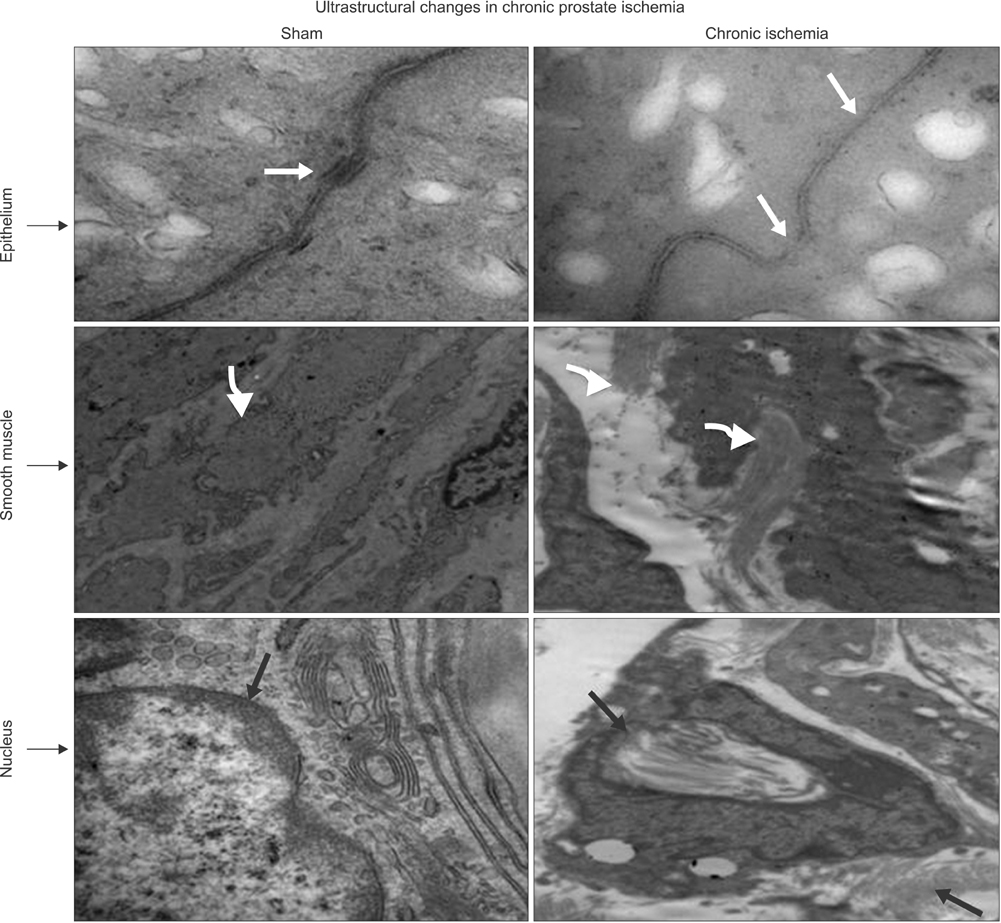
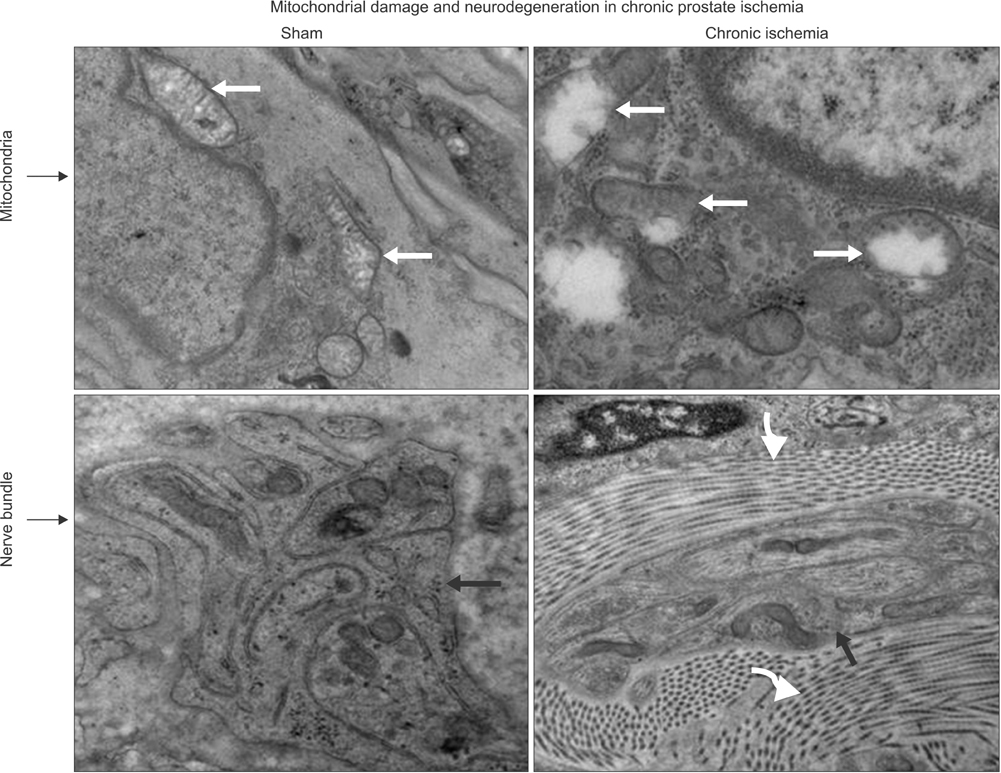
Lipid peroxidation was found in SMCs exposed to hypoxia and in all cell types exposed to oxidative stress. We identified protein oxidation in ECs exposed to hypoxia and in all cell types exposed to oxidative stress. Markers indicating oxidative damage were present in chronically ischemic rabbit prostate tissue. These reactions were associated with DNA damage. Prostate ischemia resulted in epithelial atrophy, loss of smooth muscle, and diffuse fibrosis. TEM showed swollen mitochondria with degraded cristae, loss of membrane, loss of Golgi bodies, degenerated nerves, and disrupted cell-to-cell junctions.
CONCLUSIONS
Human prostate cells exhibited differential reactions to hypoxia and oxidative stress with widespread DNA damage. Structural modifications in ischemic prostate tissue were similar to those in cells exposed to oxidative stress. Structural changes due to ischemia and oxidative stress may contribute to prostatic noncompliance in aging men.
Keyword
MeSH Terms
-
Animals
Anoxia/*complications
Atherosclerosis/complications
Biomarkers
Cells, Cultured
DNA Damage
Disease Models, Animal
Epithelial Cells/ultrastructure
Fibrosis
Humans
Ischemia/*complications
Lipid Peroxidation
Male
Myocytes, Smooth Muscle/ultrastructure
Nerve Degeneration
*Oxidative Stress
Prostate/*anatomy & histology/*cytology
Rabbits
Stromal Cells/ultrastructure
Urinary Bladder Neck Obstruction/complications
Biomarkers
Figure
Reference
-
1. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984; 132:474–479.2. Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology. 2001; 58:6 Suppl 1. 5–16.3. Madersbacher S, Pycha A, Schatzl G, Mian C, Klingler CH, Marberger M. The aging lower urinary tract: a comparative urodynamic study of men and women. Urology. 1998; 51:206–212.4. Barry MJ, Cockett AT, Holtgrewe HL, McConnell JD, Sihelnik SA, Winfield HN. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol. 1993; 150(2 Pt 1):351–358.5. Bosch JL, Kranse R, van Mastrigt R, Schroder FH. Reasons for the weak correlation between prostate volume and urethral resistance parameters in patients with prostatism. J Urol. 1995; 153(3 Pt 1):689–693.6. Girman CJ, Jacobsen SJ, Guess HA, Oesterling JE, Chute CG, Panser LA, et al. Natural history of prostatism: relationship among symptoms, prostate volume and peak urinary flow rate. J Urol. 1995; 153:1510–1515.7. Diokno AC, Brown MB, Goldstein NG, Herzog AR. Urinary flow rates and voiding pressures in elderly men living in a community. J Urol. 1994; 151:1550–1553.8. Eckhardt MD, van Venrooij GE, Boon TA. Symptoms, prostate volume, and urodynamic findings in elderly male volunteers without and with LUTS and in patients with LUTS suggestive of benign prostatic hyperplasia. Urology. 2001; 58:966–971.9. Gup DI, Shapiro E, Baumann M, Lepor H. Contractile properties of human prostate adenomas and the development of infravesical obstruction. Prostate. 1989; 15:105–114.10. Hutchison A, Farmer R, Verhamme K, Berges R, Navarrete RV. The efficacy of drugs for the treatment of LUTS/BPH, a study in 6 European countries. Eur Urol. 2007; 51:207–215.11. Ichiyanagi O, Sasagawa I, Suzuki Y, Ishigooka M, Nakada T. Relation between urethral elasticity and bladder outlet obstruction and histologic composition of the prostate in patients with benign prostatic hyperplasia. Urology. 1999; 53:1149–1153.12. Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G. The long-term outcome of medical therapy for BPH. Eur Urol. 2007; 51:1522–1533.13. McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003; 349:2387–2398.14. Berger AP, Bartsch G, Deibl M, Alber H, Pachinger O, Fritsche G, et al. Atherosclerosis as a risk factor for benign prostatic hyperplasia. BJU Int. 2006; 98:1038–1042.15. De EJ, Hou P, Estrera AL, Sdringola S, Kramer LA, Graves DE, et al. Pelvic ischemia is measurable and symptomatic in patients with coronary artery disease: a novel application of dynamic contrast-enhanced magnetic resonance imaging. J Sex Med. 2008; 5:2635–2645.16. Tarcan T, Azadzoi KM, Siroky MB, Goldstein I, Krane RJ. Agerelated erectile and voiding dysfunction: the role of arterial insufficiency. Br J Urol. 1998; 82:Suppl 1. 26–33.17. Azadzoi KM, Chen BG, Radisavljevic ZM, Siroky MB. Molecular reactions and ultrastructural damage in the chronically ischemic bladder. J Urol. 2011; 186:2115–2122.18. Azadzoi KM, Radisavljevic ZM, Golabek T, Yalla SV, Siroky MB. Oxidative modification of mitochondrial integrity and nerve fiber density in the ischemic overactive bladder. J Urol. 2010; 183:362–369.19. Azadzoi KM, Yalla SV, Siroky MB. Oxidative stress and neurodegeneration in the ischemic overactive bladder. J Urol. 2007; 178:710–715.20. Azadzoi KM, Babayan RK, Kozlowski R, Siroky MB. Chronic ischemia increases prostatic smooth muscle contraction in the rabbit. J Urol. 2003; 170(2 Pt 1):659–663.21. Azadzoi KM, Yalla SV, Siroky MB. Human bladder smooth muscle cell damage in disturbed oxygen tension. Urology. 2011; 78:967.e9. 967.e15.22. Kozlowski R, Kershen RT, Siroky MB, Krane RJ, Azadzoi KM. Chronic ischemia alters prostate structure and reactivity in rabbits. J Urol. 2001; 165:1019–1026.23. Zarifpour M, Nomiya M, Sawada N, Andersson KE. Protective effect of tadalafil on the functional and structural changes of the rat ventral prostate caused by chronic pelvic ischemia. Prostate. 2015; 75:233–241.24. Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB. Overactivity and structural changes in the chronically ischemic bladder. J Urol. 1999; 162:1768–1778.25. Pinggera GM, Mitterberger M, Steiner E, Pallwein L, Frauscher F, Aigner F, et al. Association of lower urinary tract symptoms and chronic ischaemia of the lower urinary tract in elderly women and men: assessment using colour Doppler ultrasonography. BJU Int. 2008; 102:470–474.26. Wehrberger C, Temml C, Gutjahr G, Berger I, Rauchenwald M, Ponholzer A, et al. Is there an association between lower urinary tract symptoms and cardiovascular risk in men? A cross sectional and longitudinal analysis. Urology. 2011; 78:1063–1067.27. Kim S, Jeong JY, Choi YJ, Kim DH, Lee WK, Lee SH, et al. Association between lower urinary tract symptoms and vascular risk factors in aging men: The Hallym Aging Study. Korean J Urol. 2010; 51:477–482.28. Ponholzer A, Temml C, Wehrberger C, Marszalek M, Madersbacher S. The association between vascular risk factors and lower urinary tract symptoms in both sexes. Eur Urol. 2006; 50:581–586.29. Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003; 278:8516–8525.30. Voss P, Siems W. Clinical oxidation parameters of aging. Free Radic Res. 2006; 40:1339–1349.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The role of oxidative stress and hypoxia in renal disease
- Neuroprotection in the Newborn Infant
- Oxidative Stress, Diet and Prostate Cancer
- Effect of Hypoxia-ischemia on c-fos Expression in the Neonatal Rat Brain
- Hepatic ischemia-reperfusion injury with respect to oxidative stress and inflammatory response: a narrative review