Immune Netw.
2015 Dec;15(6):304-312. 10.4110/in.2015.15.6.304.
Anti-inflammatory Effect of Alloferon on Ovalbumin-induced Asthma
- Affiliations
-
- 1Department of Anatomy, Seoul National University College of Medicine, Seoul 03080, Korea. genius29@snu.ac.kr
- 2Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul 03080, Korea.
- KMID: 2132704
- DOI: http://doi.org/10.4110/in.2015.15.6.304
Abstract
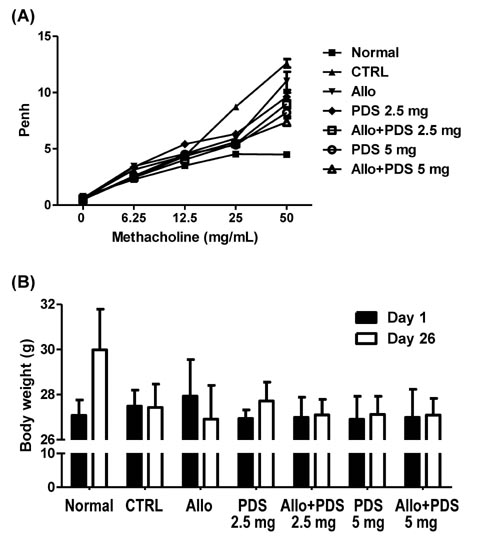
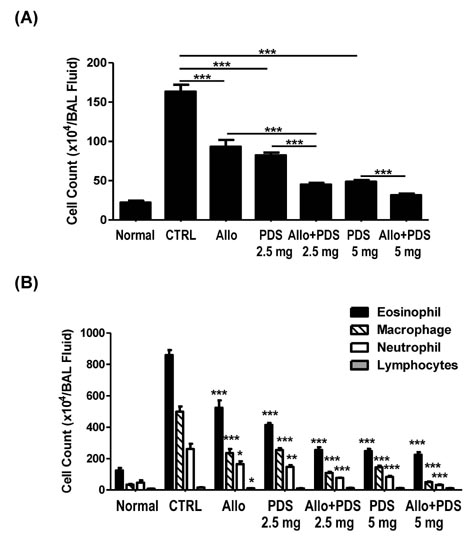
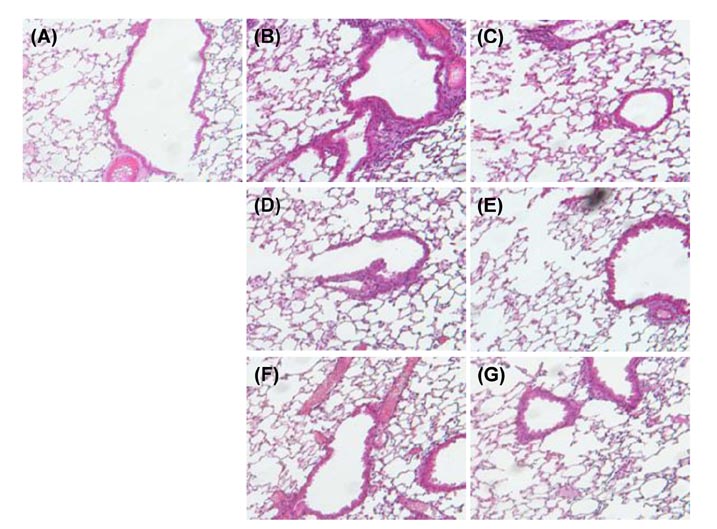
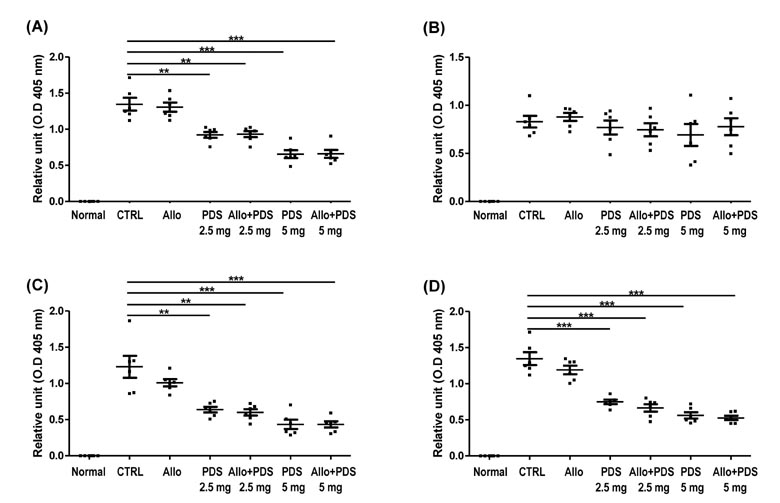
- Asthma is a well-known inflammatory lung disease; however, the specific underlying mechanism is largely unknown. We previously demonstrated that alloferon effectively downregulates pulmonary inflammation. In this study, we examined whether alloferon has a therapeutic effect on asthma. Alloferon remarkably decreased the number of eosinophils, macrophages, and neutrophils in the bronchoalveolar lavage fluid (BALF) from ovalbumin (OVA)-induced asthma mice. It was synergistically decreased with 2.5 mg/kg prednisolone (PDA). Inflammatory cell infiltration around the bronchioles and in the alveolus of OVA-induced asthma mice was effectively prevented by alloferon alone and combined treatment with alloferon and PDS. The production of IL-5 and IL-17 was decreased by alloferon alone and combined treatment with alloferon and PDS. There was no change the level of total immunoglobulin (Ig) following alloferon administration; however, total Ig was decreased by PDS. IgG2a levels were not changed by either alloferon alone or alloferon in combination with PDS. However, the levels of OVA-specific IgG1 and IgE were decreased by alloferon and PDS. In conclusion, our results suggest that a combination of alloferon and prednisolone is effective for the treatment of asthma, as it prevents inflammatory cell infiltration via the down-regulation of IL-5 and IL-17 production and decreases IgG1 and IgE production via the suppression of T helper type 2 immune response.
Keyword
MeSH Terms
-
Animals
Asthma*
Bronchioles
Bronchoalveolar Lavage Fluid
Down-Regulation
Eosinophils
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Interleukin-17
Interleukin-5
Lung Diseases
Macrophages
Mice
Neutrophils
Ovalbumin
Pneumonia
Prednisolone
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Interleukin-17
Interleukin-5
Ovalbumin
Prednisolone
Figure
Reference
-
1. Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003; 111:215–225.
Article2. Redington AE, Howarth PH. Airway wall remodelling in asthma. Thorax. 1997; 52:310–312.
Article3. Belvisi MG, Brown TJ, Wicks S, Foster ML. New Glucocorticosteroids with an improved therapeutic ratio? Pulm Pharmacol Ther. 2001; 14:221–227.
Article4. Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998; 50:515–596.5. Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999; 54:825–857.
Article6. Barnes PJ. Novel approaches and targets for treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 160:S72–S79.
Article7. Borish LC, Nelson HS, Lanz MJ, Claussen L, Whitmore JB, Agosti JM, Garrison L. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J Respir Crit Care Med. 1999; 160:1816–1823.8. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophil, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000; 356:2144–2148.
Article9. Greenfeder S, Umland SP, Cuss FM, Chapman RW, Egan RW. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir Res. 2001; 2:71–79.10. Wills-Karp M. The gene encoding interleukin-13: a susceptibility locus for asthma and related traits. Respir Res. 2000; 1:19–23.
Article11. Nakajima H, Hirose K. Role of IL-23 and Th17 Cells in Airway Inflammation in Asthma. Immune Netw. 2010; 10:1–4.
Article12. Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003; 28:42–50.
Article13. Oda N, Canelos PB, Essayan DM, Plunkett BA, Myers AC, Huang SK. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am J Respir Crit Care Med. 2005; 171:12–18.
Article14. Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005; 201:233–240.
Article15. McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007; 8:1390–1397.
Article16. Chernysh S, Kim SI, Bekker G, Pleskach VA, Filatova NA, Anikin VB, Platonov VG, Bulet P. Antiviral and antitumor peptides from insects. Proc Natl Acad Sci USA. 2002; 99:12628–12632.
Article17. Lee N, Bae S, Kim H, Kong JM, Kim HR, Cho BJ, Kim SJ, Seok SH, Hwang YI, Kim S, Kang JS, Lee WJ. Inhibition of lytic reactivation of Kaposi's sarcoma-associated herpesvirus by alloferon. Antivir Ther. 2011; 16:17–26.
Article18. Bae S, Oh K, Kim H, Kim Y, Kim HR, Hwang YI, Lee DS, Kang JS, Lee WJ. The effect of alloferon on the enhancement of NK cell cytotoxicity against cancer via the up-regulation of perforin/granzyme B secretion. Immunobiology. 2013; 218:1026–1033.
Article19. Kim Y, Lee SK, Bae S, Kim H, Park Y, Chu NK, Kim SG, Kim HR, Hwang YI, Kang JS, Lee WJ. The anti-inflammatory effect of alloferon on UVB-induced skin inflammation through the down-regulation of pro-inflammatory cytokines. Immunol Lett. 2013; 149:110–118.
Article20. Kim H, Im JP, Kim JS, Kang JS, Lee WJ. Alloferon Alleviates Dextran Sulfate Sodium-induced Colitis. Immune Netw. 2015; 15:135–141.
Article21. Sitkauskiene B, Johansson AK, Sergejeva S, Lundin S, Sjostrand M, Lotvall J. Regulation of bone marrow and airway CD34+ eosinophils by interleukin-5. Am J Respir Cell Mol Biol. 2004; 30:367–378.
Article22. Vignola AM, Chanez P, Chiappara G, Siena L, Merendino A, Reina C, Gagliardo R, Profita M, Bousquet J, Bonsignore G. Evaluation of apoptosis of eosinophils, macrophages,and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999; 103:563–573.
Article23. Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyper-responsiveness. Am J Respir Crit Care Med. 2009; 180:720–730.
Article24. Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. JAllergy Clin Immunol. 2003; 111:1293–1298.
Article25. Nagao K, Akabane H, Masuda T, Komai M, Tanaka H, Nagai H. Effect of MX-68 on airway inflammation and hyperresponsiveness in mice and guinea-pigs. J Pharm Pharmacol. 2004; 56:187–196.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alloferon Alleviates Dextran Sulfate Sodium-induced Colitis
- Suppressive Effect of Carnosol on Ovalbumin-Induced Allergic Asthma
- Suppressive Effect of 4-Hydroxy-2-(4-Hydroxyphenethyl) Isoindoline-1,3-Dione on Ovalbumin-Induced Allergic Asthma
- 4-CMTB Ameliorates Ovalbumin-Induced Allergic Asthma through FFA2 Activation in Mice
- Suppressive Effect of CYM50358 S1P4 Antagonist on Mast Cell Degranulation and Allergic Asthma in Mice