Allergy Asthma Immunol Res.
2012 Jan;4(1):37-45. 10.4168/aair.2012.4.1.37.
Role of Angiogenic Factors in Airway Remodeling in an Allergic Rhinitis Murine Model
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Otorhinolaryngology, Seoul National University College of Medicine, Seoul, Korea. ygmin312@dreamwiz.com
- 3Sensory Organ Research Institute, Seoul National University Medical Research Center, Seoul, Korea.
- 4Department of Immunology, Seoul National University Graduate School of Medicine, Seoul, Korea.
- 5Department of Otolaryngology-Head and Neck Surgery, Seoul National University Boramae Hospital, Seoul, Korea.
- 6Department of Otorhinolaryngology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2130377
- DOI: http://doi.org/10.4168/aair.2012.4.1.37
Abstract
- PURPOSE
There is growing evidence that nasal airway remodeling occurs in allergic rhinitis (AR). Although angiogenesis is an important component of airway remodeling in asthma, its involvement in AR has been little studied. Furthermore, information regarding the role of potent angiogenic factors, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), in the nasal airway remodeling process is limited. This study was conducted to investigate the role of VEGF and PDGF in nasal airway remodeling, and to assess the preventive effects of anti-angiogenic drugs on this process in a murine AR model.
METHODS
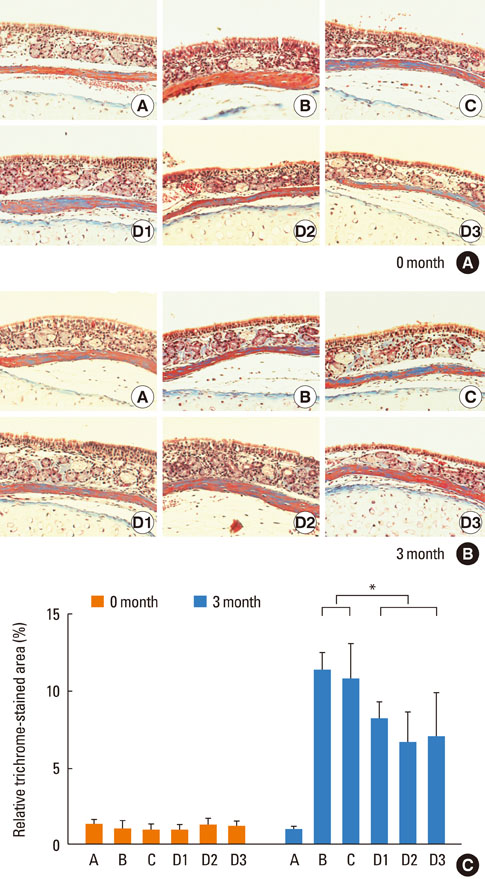
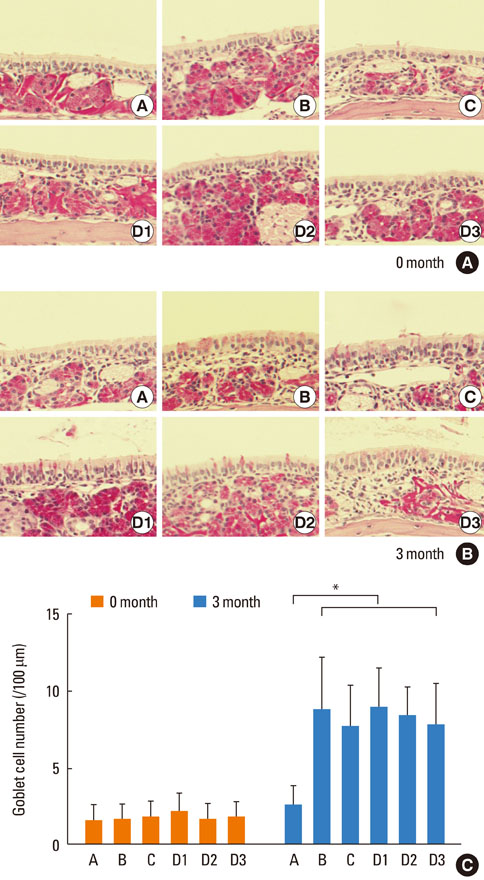
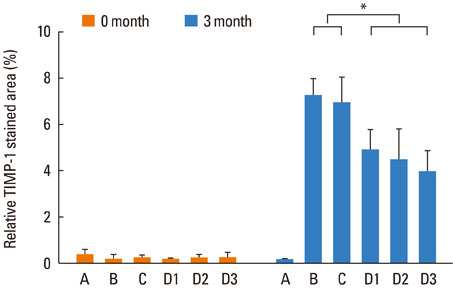
Mice were systemically sensitized and subjected to inhalation of ovalbumin (OVA) twice a week for 3 months. Control mice were challenged with phosphate buffered saline, while the treatment group received SU1498, a VEGF receptor inhibitor, and/or AG1296, a PDGF receptor inhibitor, via intraperitoneal injection 4 hours prior to each OVA inhalation. Staining using hematoxylin and eosin, Masson's trichrome, and periodic acid-Schiff were separately performed to assess eosinophil infiltration, subepithelial fibrosis, and goblet cell hyperplasia, respectively, in the nasal airway. Immunohistochemical staining for matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) was also conducted.
RESULTS
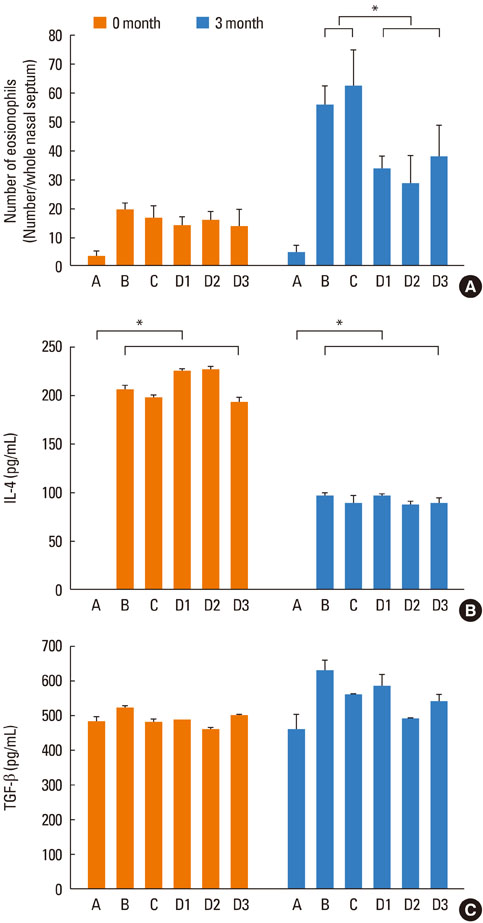
Repetitive intranasal inhalation of OVA resulted in significant increases in eosinophil infiltration, subepithelial fibrosis, goblet cell count, and MMP-9/TIMP-1 expression. Administration of SU1498 or AG1296 prevented these abnormal responses.
CONCLUSIONS
The results of this study suggest that a causal relationship may exist between angiogenic factors and nasal airway remodeling in AR. Inhibition of VEGF or PDGF receptors may, in turn, suppress the remodeling process through the regulation of MMP-9/TIMP-1 expression.
Keyword
MeSH Terms
-
Airway Remodeling
Angiogenesis Inducing Agents
Angiogenesis Inhibitors
Animals
Asthma
Cinnamates
Eosine Yellowish-(YS)
Eosinophils
Fibrosis
Goblet Cells
Hematoxylin
Hyperplasia
Inhalation
Injections, Intraperitoneal
Matrix Metalloproteinase 9
Mice
Nose
Ovalbumin
Ovum
Platelet-Derived Growth Factor
Receptors, Platelet-Derived Growth Factor
Receptors, Vascular Endothelial Growth Factor
Rhinitis
Rhinitis, Allergic, Perennial
Tissue Inhibitor of Metalloproteinase-1
Tyrphostins
Vascular Endothelial Growth Factor A
Angiogenesis Inducing Agents
Angiogenesis Inhibitors
Cinnamates
Eosine Yellowish-(YS)
Hematoxylin
Matrix Metalloproteinase 9
Ovalbumin
Platelet-Derived Growth Factor
Receptors, Platelet-Derived Growth Factor
Receptors, Vascular Endothelial Growth Factor
Tissue Inhibitor of Metalloproteinase-1
Tyrphostins
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Compalati E, Ridolo E, Passalacqua G, Braido F, Villa E, Canonica GW. The link between allergic rhinitis and asthma: the united airways disease. Expert Rev Clin Immunol. 2010. 6:413–423.2. Pawankar R, Takizawa R. Revisiting the link between allergic rhinitis and asthma. Curr Allergy Asthma Rep. 2007. 7:77–78.3. Bai TR. Evidence for airway remodeling in chronic asthma. Curr Opin Allergy Clin Immunol. 2010. 10:82–86.4. Halwani R, Al-Muhsen S, Hamid Q. Airway remodeling in asthma. Curr Opin Pharmacol. 2010. 10:236–245.5. Warner SM, Knight DA. Airway modeling and remodeling in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol. 2008. 8:44–48.6. Lim YS, Won TB, Shim WS, Kim YM, Kim JW, Lee CH, Min YG, Rhee CS. Induction of airway remodeling of nasal mucosa by repetitive allergen challenge in a murine model of allergic rhinitis. Ann Allergy Asthma Immunol. 2007. 98:22–31.7. Nakaya M, Dohi M, Okunishi K, Nakagome K, Tanaka R, Imamura M, Yamamoto K, Kaga K. Prolonged allergen challenge in murine nasal allergic rhinitis: nasal airway remodeling and adaptation of nasal airway responsiveness. Laryngoscope. 2007. 117:881–885.8. Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003. 111:291–297.9. Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy. 2010. 65:946–958.10. Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Panizzolo C, Zanin ME, Zuin R, Maestrelli P, Fabbri LM, Saetta M. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006. 174:975–981.11. Feltis BN, Wignarajah D, Zheng L, Ward C, Reid D, Harding R, Walters EH. Increased vascular endothelial growth factor and receptors: relationship to angiogenesis in asthma. Am J Respir Crit Care Med. 2006. 173:1201–1207.12. Tigani B, Cannet C, Karmouty-Quintana H, Blé FX, Zurbruegg S, Schaeublin E, Fozard JR, Beckmann N. Lung inflammation and vascular remodeling after repeated allergen challenge detected noninvasively by MRI. Am J Physiol Lung Cell Mol Physiol. 2007. 292:L644–L653.13. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003. 9:669–676.14. Hlushchuk R, Baum O, Gruber G, Wood J, Djonov V. The synergistic action of a VEGF-receptor tyrosine-kinase inhibitor and a sensitizing PDGF-receptor blocker depends upon the stage of vascular maturation. Microcirculation. 2007. 14:813–825.15. Ingram JL, Bonner JC. EGF and PDGF receptor tyrosine kinases as therapeutic targets for chronic lung diseases. Curr Mol Med. 2006. 6:409–421.16. Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, Elias JA. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004. 10:1095–1103.17. Yamashita N, Sekine K, Miyasaka T, Kawashima R, Nakajima Y, Nakano J, Yamamoto T, Horiuchi T, Hirai K, Ohta K. Platelet-derived growth factor is involved in the augmentation of airway responsiveness through remodeling of airways in diesel exhaust particulate-treated mice. J Allergy Clin Immunol. 2001. 107:135–142.18. Lee KS, Min KH, Kim SR, Park SJ, Park HS, Jin GY, Lee YC. Vascular endothelial growth factor modulates matrix metalloproteinase-9 expression in asthma. Am J Respir Crit Care Med. 2006. 174:161–170.19. Lee KS, Kim SR, Park SJ, Min KH, Lee KY, Choe YH, Park SY, Chai OH, Zhang X, Song CH, Lee YC. Mast cells can mediate vascular permeability through regulation of the PI3K-HIF-1alpha-VEGF axis. Am J Respir Crit Care Med. 2008. 178:787–797.20. Laschke MW, Elitzsch A, Vollmar B, Vajkoczy P, Menger MD. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum Reprod. 2006. 21:262–268.21. Zhang F, Tang Z, Hou X, Lennartsson J, Li Y, Koch AW, Scotney P, Lee C, Arjunan P, Dong L, Kumar A, Rissanen TT, Wang B, Nagai N, Fons P, Fariss R, Zhang Y, Wawrousek E, Tansey G, Raber J, Fong GH, Ding H, Greenberg DA, Becker KG, Herbert JM, Nash A, Yla-Herttuala S, Cao Y, Watts RJ, Li X. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA. 2009. 106:6152–6157.22. Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004. 18:338–340.23. Farhadi MR, Capelle HH, Erber R, Ullrich A, Vajkoczy P. Combined inhibition of vascular endothelial growth factor and platelet-derived growth factor signaling: effects on the angiogenesis, microcirculation, and growth of orthotopic malignant gliomas. J Neurosurg. 2005. 102:363–370.24. Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010. 20:161–168.25. Lei F, Zhu D, Sun J, Dong Z. Effects of minimal persistent inflammation on nasal mucosa of experimental allergic rhinitis. Am J Rhinol Allergy. 2010. 24:e23–e28.26. Shin HW, Han DH, Lim YS, Kim HJ, Kim DY, Lee CH, Min YG, Rhee CS. Nonasthmatic nasal polyposis patients with allergy exhibit greater epithelial MMP positivity. Otolaryngol Head Neck Surg. 2009. 141:442–447.27. Tedeschi A, Asero R, Marzano AV, Lorini M, Fanoni D, Berti E, Cugno M. Plasma levels and skin-eosinophil-expression of vascular endothelial growth factor in patients with chronic urticaria. Allergy. 2009. 64:1616–1622.28. Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004. 25:477–482.29. Choi GS, Park HJ, Hur GY, Choi SJ, Shin SY, Ye YM, Park HS. Vascular endothelial growth factor in allergen-induced nasal inflammation. Clin Exp Allergy. 2009. 39:655–661.30. Millette E, Rauch BH, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB transactivates the fibroblast growth factor receptor to induce proliferation in human smooth muscle cells. Trends Cardiovasc Med. 2006. 16:25–28.31. Ito I, Fixman ED, Asai K, Yoshida M, Gounni AS, Martin JG, Hamid Q. Platelet-derived growth factor and transforming growth factor-beta modulate the expression of matrix metalloproteinases and migratory function of human airway smooth muscle cells. Clin Exp Allergy. 2009. 39:1370–1380.32. Bach MK, Brashler JR, Stout BK, Johnson HG, Sanders ME, Lin AH, Gorman RR, Bienkowski MJ, Ishizaka T. Activation of human eosinophils by platelet-derived growth factor. Int Arch Allergy Immunol. 1992. 97:121–129.33. Rogers DF. The airway goblet cell. Int J Biochem Cell Biol. 2003. 35:1–6.34. Izuhara K, Ohta S, Shiraishi H, Suzuki S, Taniguchi K, Toda S, Tanabe T, Yasuo M, Kubo K, Hoshino T, Aizawa H. The mechanism of mucus production in bronchial asthma. Curr Med Chem. 2009. 16:2867–2875.35. Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol. 1998. 102:783–788.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Angiogenic Factors Play a Significant Role in Nasal Airway Remodeling in Allergic Rhinitis
- Roles of Periostin in Symptom Manifestation and Airway Remodeling in a Murine Model of Allergic Rhinitis
- Unified Airway in ENT Field
- Allergic Rhinitis Mouse Model
- Allergic rhinitis, sinusitis and asthma: evidence for respiratory system integration