Allergy Asthma Immunol Res.
2012 Jan;4(1):24-30. 10.4168/aair.2012.4.1.24.
Decreased Expression of FOXP3 in Nasal Polyposis
- Affiliations
-
- 1Rhinology and Allergy, Nippon Medical School, Tokyo, Japan. pawankar.ruby@gmail.com
- 2Department of Otolaryngology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
- KMID: 2130375
- DOI: http://doi.org/10.4168/aair.2012.4.1.24
Abstract
- PURPOSE
The pathogenesis of nasal polyposis (NP) is unclear. Eosinophils and mast cells are considered to play important roles in this process. In addition, the levels of Th2-type cells are increased, irrespective of the atopic status of the patient with NP. In this context, we and others have shown high levels of thymus and activation-related chemokine/CCL17, macrophage-derived chemokine, eotaxin, and RANTES in patients with NP. Forkhead box P3 (FOXP3) plays a key role in CD4+CD25+ regulatory T-cell function and represents a specific marker for regulatory T cells (Tregs). Decreased expression of FOXP3 has been reported in allergic diseases. The present study was designed to evaluate the presence and potential roles of Tregs, defined by the expression of FOXP3 protein, in NP.
METHODS
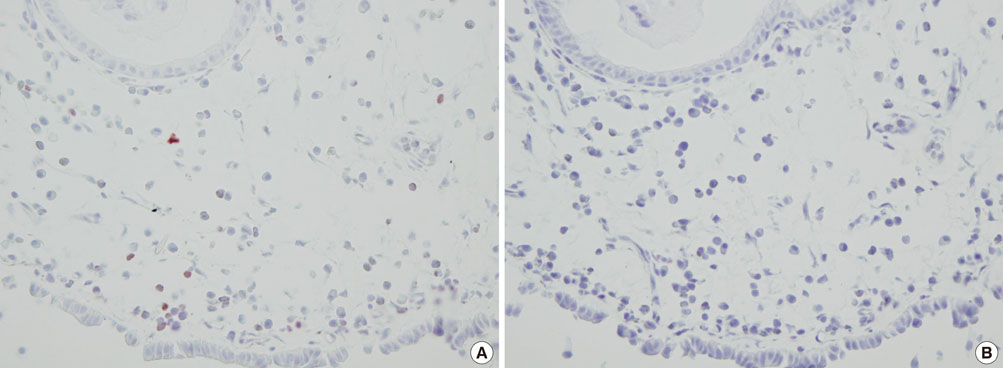
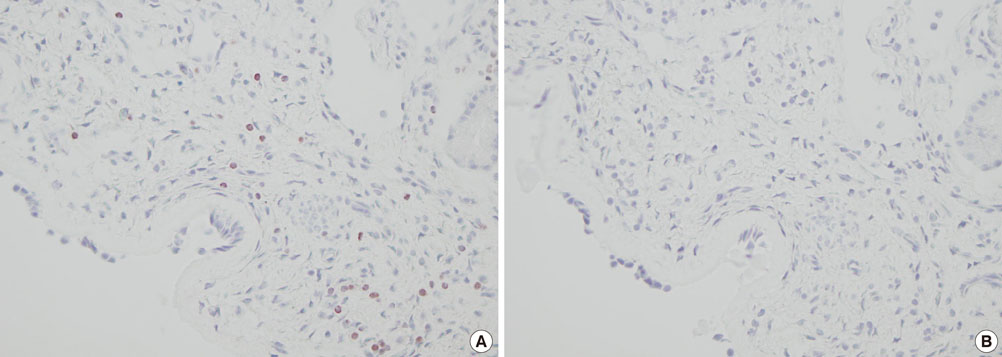
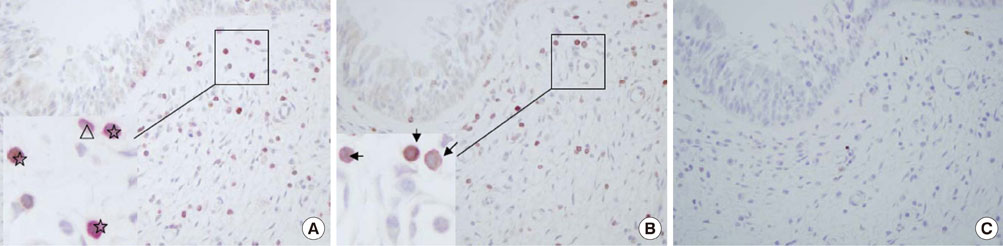
Using immunohistochemistry, we estimated the numbers of FOXP3+ cells in the epithelium and lamina propria of the NPs of 17 patients with chronic rhinosinusitis with NP and the nasal mucosa of 15 patients with allergic rhinitis (AR). The number of FOXP3+ cells in NPs was compared with that in the nasal mucosa of patients with AR, and the numbers of FOXP3+ cells in atopic and non-atopic NP were also compared.
RESULTS
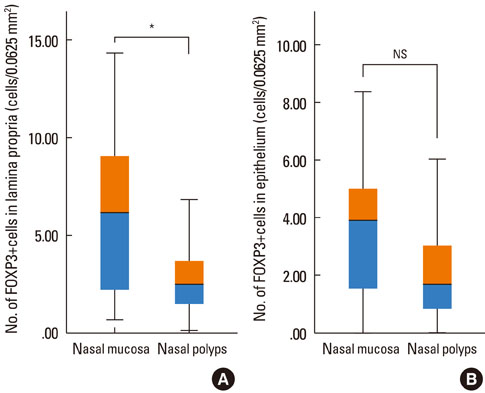
The number of FOXP3+ cells in the lamina propria of patients with NP was significantly lower than that in the nasal mucosa of the AR patients (2.79 vs. 5.99, P=0.008). There was no statistically significant difference noted for the numbers of FOXP3+ cells between the epithelium of the NP and the nasal mucosa (3.60 vs. 2.39, P=0.180). Furthermore, the numbers of CD4+FOXP3+ cells were lower in NPs than in the allergic nasal mucosa. There was no difference in the number of FOXP3+ cells between the atopic and non-atopic NP patients.
CONCLUSIONS
Fewer Tregs (i.e., decreased FOXP3 expression) are found in NPs than in the nasal mucosa of AR patients. As the severity of eosinophilic, Th2-type inflammation and the levels of inflammatory mediators are much higher in NPs than in the nasal mucosa of AR patients, an inverse co-relationship may exist between these parameters and the number of Tregs. The deficiency of Tregs in NP may account for the more pronounced Th2-type inflammation seen in these patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Pearlman AN, Chandra RK, Chang D, Conley DB, Tripathi-Peters A, Grammer LC, Schleimer RT, Kern RC. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009. 23:145–148.2. Pawankar R, Nonaka M. Inflammatory mechanisms and remodeling in chronic rhinosinusitis and nasal polyps. Curr Allergy Asthma Rep. 2007. 7:202–208.3. Sánchez-Segura A, Brieva JA, Rodríguez C. T lymphocytes that infiltrate nasal polyps have a specialized phenotype and produce a mixed TH1/TH2 pattern of cytokines. J Allergy Clin Immunol. 1998. 102:953–960.4. Hamilos DL, Leung DY, Huston DP, Kamil A, Wood R, Hamid Q. GM-CSF, IL-5 and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis (NP). Clin Exp Allergy. 1998. 28:1145–1152.5. Patou J, Gevaert P, Van Zele T, Holtappels G, van Cauwenberge P, Bachert C. Staphylococcus aureus enterotoxin B, protein A, and lipoteichoic acid stimulations in nasal polyps. J Allergy Clin Immunol. 2008. 121:110–115.6. Fukumoto A, Nonaka M, Ogihara N, Pawankar R. Induction of TARC production by lipopolysaccharide and interleukin-4 in nasal fibroblasts. Int Arch Allergy Immunol. 2008. 145:291–297.7. Bernstein JM, Allen C, Rich G, Dryja D, Bina P, Reiser R, Ballow M, Wilding GE. Further observations on the role of Staphylococcus aureus exotoxins and IgE in the pathogenesis of nasal polyposis. Laryngoscope. 2011. 121:647–655.8. Kimura S, Pawankar R, Mori S, Nonaka M, Masuno S, Yagi T, Okubo K. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011. 3:186–193.9. Ling Y, Cao XS, Yu ZQ, Luo GH, Bai X, Su J, Dai L, Ruan CG. Alterations of CD4+ CD25+ regulatory T cells in patients with idiopathic thrombocytopenic purpura. Zhonghua Xue Ye Xue Za Zhi. 2007. 28:184–188.10. Pekiner FN, Demirel GY, Gümrü B, Ozbayrak S. Serum cytokine and T regulatory cell levels in patients with burning mouth syndrome. J Oral Pathol Med. 2008. 37:528–534.11. Wang J, Zhao R, Zhang F, Li J, Huo B, Cao Y, Dou K. Control of allograft rejection in mice by applying a novel neuropeptide, cortistatin. Adv Ther. 2008. 25:1331–1341.12. Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB, Brusselle GG, Joos GF, Tournoy KG. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009. 64:1539–1546.13. Paik Y, Dahl M, Fang D, Calhoun K. Update: the role of FoxP3 in allergic disease. Curr Opin Otolaryngol Head Neck Surg. 2008. 16:275–279.14. Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009. 10:689–695.15. Dutsch-Wicherek M, Tomaszewska R, Lazar A, Strek P, Wicherek L, Kijowski J, Majka M. The presence of B7-H4+ macrophages and CD25+CD4+ and FOXP3+ regulatory T cells in the microenvironment of nasal polyps - a preliminary report. Folia Histochem Cytobiol. 2010. 48:611–617.16. Mason DY, Sammons R. Alkaline phosphatase and peroxidase for double immunoenzymatic labelling of cellular constituents. J Clin Pathol. 1978. 31:454–460.17. Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008. 29:114–126.18. Lee JH, Yu HH, Wang LC, Yang YH, Lin YT, Chiang BL. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol. 2007. 148:53–63.19. Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, van Cauwenberge P, Bachert C. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004. 114:981–983.20. Suh KS, Park HS, Nahm DH, Kim YK, Lee YM, Park K. Role of IgG, IgA, and IgE antibodies in nasal polyp tissue: their relationships with eosinophilic infiltration and degranulation. J Korean Med Sci. 2002. 17:375–380.21. Mandron M, Ariès MF, Brehm RD, Tranter HS, Acharya KR, Charveron M, Davrinche C. Human dendritic cells conditioned with Staphylococcus aureus enterotoxin B promote TH2 cell polarization. J Allergy Clin Immunol. 2006. 117:1141–1147.22. Van Bruaene N, Pérez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, Schmidt-Weber C, Akdis C, Van Cauwenberge P, Bachert C, Gevaert P. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008. 121:1435–1441. 1441.e1–1441.e3.23. Pérez Novo CA, Jedrzejczak-Czechowicz M, Lewandowska-Polak A, Claeys C, Holtappels G, Van Cauwenberge P, Kowalski ML, Bachert C. T cell inflammatory response, Foxp3 and TNFRS18-L regulation of peripheral blood mononuclear cells from patients with nasal polyps-asthma after staphylococcal superantigen stimulation. Clin Exp Allergy. 2010. 40:1323–1332.24. Bernstein JM, Ballow M, Rich G, Allen C, Swanson M, Dmochowski J. Lymphocyte subpopulations and cytokines in nasal polyps: is there a local immune system in the nasal polyp? Otolaryngol Head Neck Surg. 2004. 130:526–535.25. Pawankar R. Nasal polyposis: an update: editorial review. Curr Opin Allergy Clin Immunol. 2003. 3:1–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epigenetic Regulation of Nasal Polyp Formation
- Significance of Susceptible Gene Expression Profiles in Nasal Polyposis
- Endotype of Eosinophilic Nasal Polyposis According to the Presence of Atopy
- Electrophysiological assessment of the concentration and attention in patient with nasal polyposis
- The Regulation of FOXP3 Expression by the Treatment of TGF-beta and the Modification of DNA Methylation in Lung Cancer Cell Lines