Susceptibility of Escherichia coli from Community-Acquired Urinary Tract Infection to Fosfomycin, Nitrofurantoin, and Temocillin in Korea
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Hanyang University, Seoul, Korea. paihyunjoo@gmail.com
- 2Department of Laboratory Medicine, Hanyang University, Seoul, Korea.
- 3Department of Clinical Microbiology, Hanyang University, Guri, Korea.
- 4Department of Internal Medicine, St. Vincent's Hospital, School of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Department of Preventive Medicine, Eulji University School of Medicine, Daejeon, Korea.
- 6Department of Internal Medicine, Gil Hospital, Gacheon University, Incheon, Korea.
- 7Department of Internal Medicine, Ajou University Hospital, Suwon, Korea.
- 8Department of Internal Medicine, Hallym University, Kangdong Sacred Heart Hospital, Seoul, Korea.
- 9Department of Internal Medicine, Daegu Patima Hospital, Daegu, Korea.
- 10Department of Internal Medicine, Dong-A University Hospital, Busan, Korea.
- 11Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea.
- 12Department of Internal Medicine, Korea University Ansan Hospital, Ansan, Korea.
- 13Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 14Department of Internal Medicine, Inha University Hospital, Incheon, Korea.
- KMID: 2129618
- DOI: http://doi.org/10.3346/jkms.2014.29.8.1178
Abstract
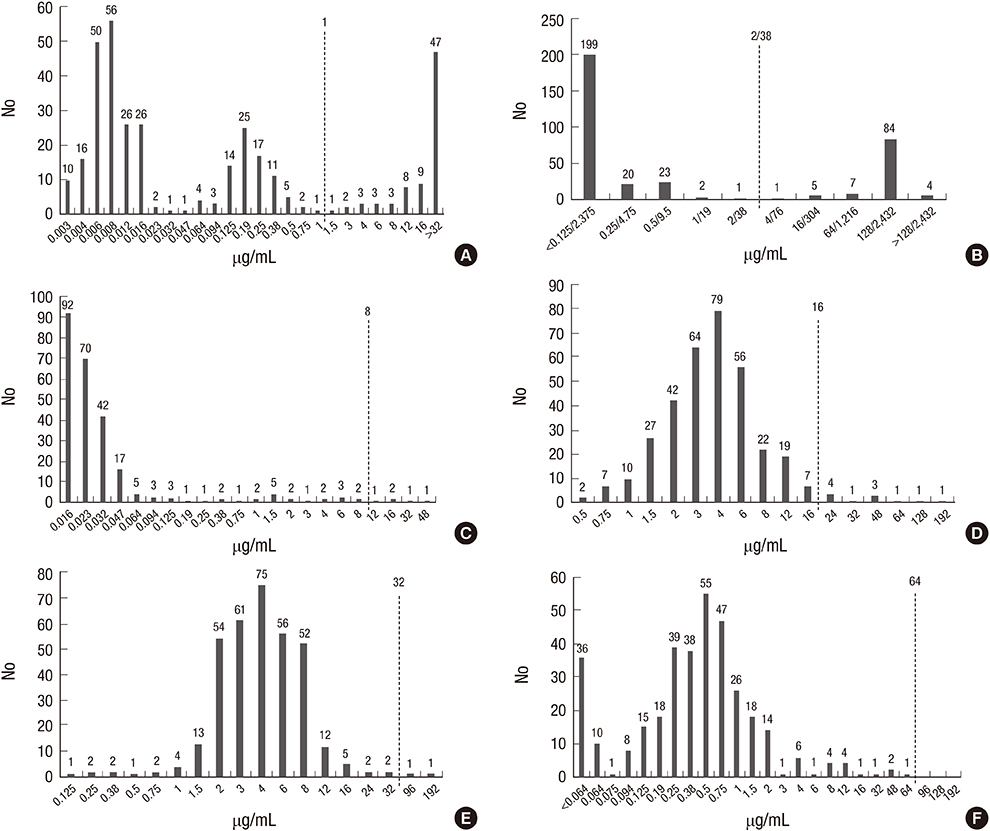
- With increase of multi-drug resistant Escherichia coli in community-acquired urinary tract infections (CA-UTI), other treatment option with a therapeutic efficacy and a low antibiotic selective pressure is necessary. In this study, we evaluated in vitro susceptibility of E. coli isolates from CA-UTI to fosfomycin (FM), nitrofurantoin (NI), temocillin (TMO) as well as trimethoprim-sulfamethoxazole (SMX), ciprofloxacin (CIP) and cefepime (FEP). The minimal inhibitory concentrations were determined by E-test or agar dilution method according to the Clinical and Laboratory Standards Institute guidelines, using 346 E. coli collected in 12 Korean hospitals from March 2010 to February 2011. FM, NI and TMO showed an excellent susceptibility profile; FM 100% (346/346), TMO 96.8% (335/346), and NI 99.4% (344/346). Conversely, resistance rates of CIP and SMX were 22% (76/346) and 29.2% (101/349), respectively. FEP still retained an activity of 98.5%. In Korea, NI and TMO in addition to FM are a good therapeutic option for uncomplicated CA-UTI, especially for lower UTI.
Keyword
MeSH Terms
-
Anti-Bacterial Agents/*administration & dosage
Cell Survival/*drug effects
Cephalosporins/administration & dosage
Ciprofloxacin/administration & dosage
Community-Acquired Infections/drug therapy/*microbiology
Dose-Response Relationship, Drug
Drug Combinations
Drug Resistance, Bacterial/drug effects
Escherichia coli/*drug effects
Escherichia coli Infections/drug therapy/*microbiology
Fosfomycin/administration & dosage
Humans
Nitrofurantoin/administration & dosage
Penicillins/administration & dosage
Republic of Korea
Sulfadoxine/administration & dosage
Treatment Outcome
Trimethoprim/administration & dosage
Urinary Tract Infections/diagnosis/*microbiology
Anti-Bacterial Agents
Cephalosporins
Ciprofloxacin
Drug Combinations
Fosfomycin
Nitrofurantoin
Penicillins
Sulfadoxine
Trimethoprim
Figure
Cited by 4 articles
-
Marker Pen Device with Concentration Gradient Nib for Antibiotic Susceptibility Testing
Yong-Gyun Jung, Young-Ran Yun, Suk-Heung Song, Wook Park
J Korean Med Sci. 2018;33(33):. doi: 10.3346/jkms.2018.33.e224.Treatment of drug resistant bacteria: new bugs, old drugs, and new therapeutic approaches
Young Hwa Choi
J Korean Med Assoc. 2014;57(10):837-844. doi: 10.5124/jkma.2014.57.10.837.The Antibiotic Susceptibility of Escherichia coli from Community-Acquired Uncomplicated Urinary Tract Infection: A Focused on Fosfomycin
Hyun-Sop Choe, Seung-Ju Lee, In Ho Chang, Tae-Hyoung Kim, Hong Chung, Jae Min Chung, Sang Don Lee, Jae Hung Jung, Ki Ho Kim, Seung Ki Min, Yong Gil Na, Hana Yoon, Ho Song Yu, Mi-Kyung Lee, Sun-Ju Lee
Urogenit Tract Infect. 2017;12(2):77-81. doi: 10.14777/uti.2017.12.2.77.Susceptibility to Fosfomycin and Nitrofurantoin of ESBL-Positive
Escherichia coli andKlebsiella pneumoniae Isolated From Urine of Pediatric Patients
Ki-Sup Park, Doo Ri Kim, Jin Yang Baek, Areum Shin, Kyung-Ran Kim, Hwanhee Park, Sohee Son, Heeyeon Cho, Yae-Jean Kim
J Korean Med Sci. 2023;38(48):e361. doi: 10.3346/jkms.2023.38.e361.
Reference
-
1. Lee SJ, Lee DS, Choe HS, Shim BS, Kim CS, Kim ME, Cho YH. Antimicrobial resistance in community-acquired urinary tract infections: results from the Korean Antimicrobial Resistance Monitoring System. J Infect Chemother. 2011; 17:440–446.2. Hooton TM. Fluoroquinolones and resistance in the treatment of uncomplicated urinary tract infection. Int J Antimicrob Agents. 2003; 22:65–72.3. Lee MY, Choi HJ, Choi JY, Song M, Song Y, Kim SW, Chang HH, Jung SI, Kim YS, Ki HK, et al. Dissemination of ST131 and ST393 community-onset, ciprofloxacin-resistant Escherichia coli clones causing urinary tract infections in Korea. J Infect. 2010; 60:146–153.4. Garau J. Other antimicrobials of interest in the era of extended-spectrum beta-lactamases: fosfomycin, nitrofurantoin and tigecycline. Clin Microbiol Infect. 2008; 14:198–202.5. Ko KS, Suh JY, Peck KR, Lee MY, Oh WS, Kwon KT, Jung DS, Lee NY, Song JH. In vitro activity of fosfomycin against ciprofloxacin-resistant or extended-spectrum beta-lactamase-producing Escherichia coli isolated from urine and blood. Diagn Microbiol Infect Dis. 2007; 58:111–115.6. Kim KY, Kim CS, Lim DH. The ciprofloxacin resistance pattern of Escherichia coli isolated from female patients with community - acquired urinary tract infection in the Jeonnam and Gwangju region for the recent 2-years. Korean J Urol. 2008; 49:540–548.7. Lee SY, Park YJ, Yu JK, Jung S, Kim Y, Jeong SH, Arakawa Y. Prevalence of acquired fosfomycin resistance among extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae clinical isolates in Korea and IS26-composite transposon surrounding fosA3. J Antimicrob Chemother. 2012; 67:2843–2847.8. Clinical and Laboratory Standard Institute. Performance standards for antimicrobial susceptibility testing: 15th informational supplement: CLSI document M100-S15. Wayne: CLSI;2005. accessed on 1 January 2005. Available at http://clsi.crg.9. Kim B, Kim J, Wie SH, Park SH, Cho YK, Lim SK, Shin SY, Yum JS, Lee JS, Kweon KT, et al. Is it acceptable to select antibiotics for the treatment of community-acquired acute cystitis based on the antibiotics susceptibility results for uropathogens from community-acquired acute pyelonephritis in Korea? Infect Chemother. 2012; 44:269–274.10. Balakrishnan I, Awad-El-Kariem FM, Aali A, Kumari P, Mulla R, Tan B, Brudney D, Ladenheim D, Ghazy A, Khan I, et al. Temocillin use in England: clinical and microbiological efficacies in infections caused by extended-spectrum and/or derepressed AmpC β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2011; 66:2628–2631.11. Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. What remains against carbapenem-resistant Enterobacteriaceae? evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents. 2011; 37:415–419.12. Andrews JM, Howe RA. BSAC Working Party on Susceptibility Testing. BSAC standardized disc susceptibility testing method (version 10). J Antimicrob Chemother. 2011; 66:2726–2757.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Ciprofloxacin Resistance Pattern of Escherichia coli Isolated from Female Patients with Community- acquired Urinary Tract Infection in the Jeonnam and Gwangju Region for the Recent 2-years
- The Antibiotic Susceptibility of Escherichia coli from Community-Acquired Uncomplicated Urinary Tract Infection: A Focused on Fosfomycin
- Susceptibility to Fosfomycin and Nitrofurantoin of ESBL-Positive Escherichia coli and Klebsiella pneumoniae Isolated From Urine of Pediatric Patients
- Change in the Annual Antibiotic Susceptibility of Escherichia coli in Community-Onset Urinary Tract Infection between 2008 and 2017 in a Tertiary Care Hospital in Korea
- Urinary Tract Infection Caused by Escherichia coli