Cancer Res Treat.
2009 Sep;41(3):164-170.
TNF-alpha Downregulates E-cadherin and Sensitizes Response to gamma-irradiation in Caco-2 Cells
- Affiliations
-
- 1Lab of Modulation of Radiobiological Response, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 2Medical & Bio-material Research Center and Department of Biology, Kangwon National University, Chuncheon, Korea.
- 3Department of Molecular and Medical Biotechnology, Kangwon National University, Korea.
- 4Biowells Inc. and Department of Biomedical Science, Hallym University, Chuncheon, Korea. chungek@hallym.ac.kr
Abstract
- PURPOSE
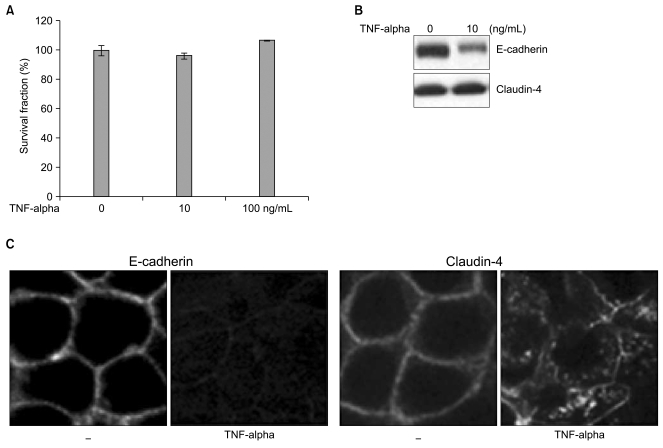
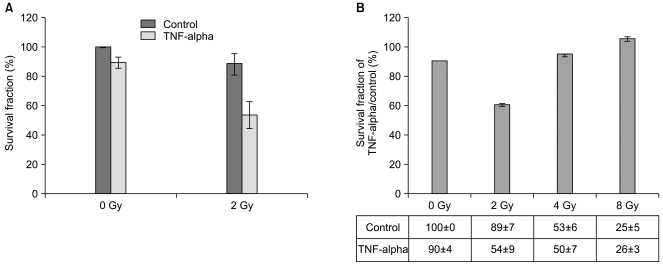
The purpose of the present study was to assess the biological effects of TNF-alpha in Caco-2 well-differentiated colon adenocarcinoma cells and to determine radiation sensitivity in order to develop TNF-alpha into a cancer therapeutic agent. MATERIALS AND METHODS: A cell viability test was conducted via a colorimetric and colony forming assay after 1 day and 3 days of incubation with TNF-alpha. Western blotting analysis and immunofluorescence staining were conducted to explore TNF-alpha-induced morphological and molecular changes in the adhesion molecules, E-cadherin and claudin-4. The effects of gamma-irradiation at a dose of 2 Gy on cell survival were evaluated by a clonogenic assay. The molecular changes in apoptosis-regulatory proteins were assessed by Western blotting. RESULTS: Caco-2 cells were highly resistant to TNF alpha-induced cell death and 2 Gy of gamma-irradiation. However, we observed the downregulation of the adherens junctional protein, E-cadherin and translocation of tight junctional protein, claudin-4 from the membrane to the cytosol induced by TNF-alpha treatment which would indicate cell-cell junction disruptions. These alterations of junctional proteins influenced the regulation of cell death in response to 2 Gy of gamma-irradiation. The combined treatment of TNF-alpha with 2 Gy of gamma-irradiation reduced the survival of Caco-2 cells by down-regulating bcl-xl and activating JNK pathways. CONCLUSION: These results suggest that TNF-alpha might be potentially applied as a therapeutic agent in order to enhance sensitivity to 2 Gy of gamma-irradiation administered in radiotherapy for the treatment of human colon cancer.
Keyword
MeSH Terms
-
Adenocarcinoma
Blotting, Western
Caco-2 Cells
Cadherins
Cell Death
Cell Survival
Claudin-4
Colon
Colonic Neoplasms
Cytosol
Down-Regulation
Fluorescent Antibody Technique
Humans
MAP Kinase Signaling System
Membranes
Proteins
Radiation Tolerance
Tumor Necrosis Factor-alpha
Cadherins
Claudin-4
Proteins
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL. Anti-TNF-alpha therapies: the next generation. Nat Rev Drug Discov. 2003; 2:736–746. PMID: 12951580.2. Younes A, Aggarwall BB. Clinical implications of the tumor necrosis factor family in benign and malignant hematologic disorders. Cancer. 2003; 98:458–467. PMID: 12879461.
Article3. Younes A, Kadin ME. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy. J Clin Oncol. 2003; 21:3526–3534. PMID: 12972530.
Article4. Jones SA, Butler RN, Sanderson IR, Wilson JW. The effect of specific caspase inhibitors on TNF-alpha and butyrate-induced apoptosis of intestinal epithelial cells. Exp Cell Res. 2004; 292:29–39. PMID: 14720504.5. Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003; 114:181–190. PMID: 12887920.
Article6. Kimura K, Bowen C, Spiegel S, Gelmann EP. Tumor necrosis factor-alpha sensitizes prostate cancer cells to gamma-irradiation-induced apoptosis. Cancer Res. 1999; 59:1606–1614. PMID: 10197636.7. Schmelz K, Wieder T, Tamm I, Muller A, Essmann F, Geilen CC, et al. Tumor necrosis factor alpha sensitizes malignant cells to chemotherapeutic drugs via the mitochondrial apoptosis pathway independently of caspase-8 and NF-kappaB. Oncogene. 2004; 23:6743–6759. PMID: 15273737.8. Anderson GM, Nakada MT, DeWitte M. Tumor necrosis factor-alpha in the pathogenesis and treatment of cancer. Curr Opin Pharmacol. 2004; 4:314–320. PMID: 15251122.9. Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, et al. Interferon gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol. 2008; 126:67–80. PMID: 17964857.10. Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, et al. Tumor necrosis factor-alpha (TNF-alpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999; 112:137–146. PMID: 9841910.
Article11. Suk K, Chang I, Kim YH, Kim S, Kim JY, Kim H, et al. Interferon gamma (IFNgamma) and tumor necrosis factor alpha synergism in ME-180 cervical cancer cell apoptosis and necrosis. IFNgamma inhibits cytoprotective NF-kappa B through STAT1/IRF-1 pathways. J Biol Chem. 2001; 276:13153–13159. PMID: 11278357.12. Angelini DJ, Hyun SW, Grigoryev DN, Garg P, Gong P, Singh IS, et al. TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol. 2006; 291:L1232–L1245. PMID: 16891393.13. Bove K, Neumann P, Gertzberg N, Johnson A. Role of ecNOS-derived NO in mediating TNF-induced endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001; 280:L914–L922. PMID: 11290515.
Article14. Eggermont AM, De Wilt JH, Ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol. 2003; 4:429–437. PMID: 12850194.
Article15. Folli S, Pelegrin A, Chalandon Y, Yao X, Buchegger F, Lienard D, et al. Tumor-necrosis factor can enhance radio-antibody uptake in human colon carcinoma xenografts by increasing vascular permeability. Int J Cancer. 1993; 53:829–836. PMID: 8449608.
Article16. Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008; 1778:660–669. PMID: 17854762.
Article17. Wacheck V, Selzer E, Gunsberg P, Lucas T, Meyer H, Thallinger C, et al. Bcl-x(L) antisense oligonucleotides radiosensitise colon cancer cells. Br J Cancer. 2003; 89:1352–1357. PMID: 14520471.
Article18. Ferreira P, Oliveira MJ, Beraldi E, Mateus AR, Nakajima T, Gleave M, et al. Loss of functional E-cadherin renders cells more resistant to the apoptotic agent taxol in vitro. Exp Cell Res. 2005; 310:99–104. PMID: 16112667.
Article19. Li L, Backer J, Wong AS, Schwanke EL, Stewart BG, Pasdar M. Bcl-2 expression decreases cadherin-mediated cell-cell adhesion. J Cell Sci. 2003; 116:3687–3700. PMID: 12890751.
Article20. Torres VA, Tapia JC, Rodriguez DA, Lladser A, Arredondo C, Leyton L, et al. E-cadherin is required for caveolin-1-mediated down-regulation of the inhibitor of apoptosis protein survivin via reduced beta-catenin-Tcf/Lef-dependent transcription. Mol Cell Biol. 2007; 27:7703–7717. PMID: 17785436.21. Gottlieb E, Vander Heiden MG, Thompson CB. Bcl-x (L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2000; 20:5680–5689. PMID: 10891504.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of TNF - alpha Gene Expression in Human Fetal Astrocytes
- CXC and CC Chemokine Expression by Intestinal Epithelial Cells in Response to Clostridium difficile Toxin A
- Expression of E-cadherin and alpha - , beta - , gamma - catenin proteins in endometrial carcinoma
- Regulation of V-cadherin Expression on Human Dermal Microvascular Endothelial Cells
- Sensitization of TNF alpha and Agonistic FAS/CD95 Antibody-Induced Apoptosis by INF gamma on Neuroblastoma Cells