Clin Exp Otorhinolaryngol.
2012 Jun;5(2):86-93. 10.3342/ceo.2012.5.2.86.
Growth Inhibitory Effect of Palatine Tonsil-derived Mesenchymal Stem Cells on Head and Neck Squamous Cell Carcinoma Cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Pusan National University School of Medicine and Medical Research Institute, Busan, Korea. voicelee@pusan.ac.kr
- KMID: 2117502
- DOI: http://doi.org/10.3342/ceo.2012.5.2.86
Abstract
OBJECTIVES
Mesenchymal stem cells (MSCs) play an important role in the development and growth of tumor cells. However, the effect of human MSCs on the growth of human tumors is not well understood. The purpose of this study is to confirm the growth effect of palatine tonsil-derived MSCs (TD-MSCs) on head and neck squamous cell carcinoma (HNSCC) cell lines and to elucidate the mechanism of their action.
METHODS
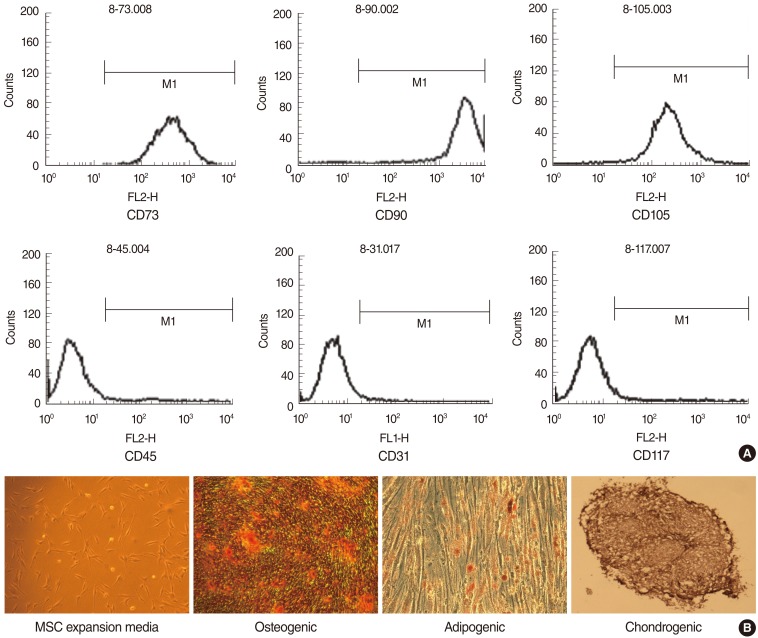
TD-MSCs were isolated from patient with chronic tonsillitis and tonsillar hypertrophy. Two human HNSCC cell lines (PNUH-12 and SNU-899) were studied and cocultured with isolated palatine tonsil-derived MSC. The growth inhibitory effect of MSCs on HNSCC cell lines was tested through methylthiazolyldiphenyl-tetrazolium (MTT) assay. The apoptosis induction effect of MSCs on cell lines was assessed with flow cytometry and reverse transcriptase (RT)-PCR.
RESULTS
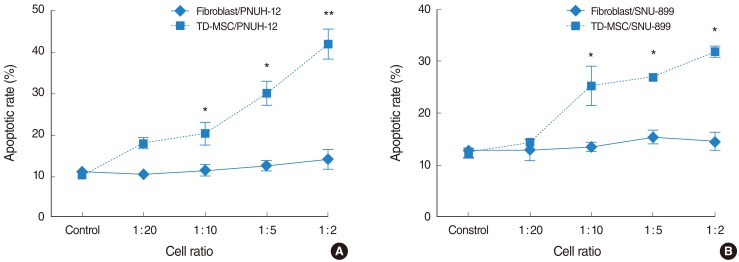
Palatine tonsil-derived MSCs exhibited a growth inhibitory effect on both cell lines. Cell cycle analysis showed an accumulation of tumor cells predominantly in G0/G1 phase with an increase in concentration of TD-MSCs, which was confirmed by increased mRNA expression of cell cycle negative regulator p21. Apoptosis of tumor cells increased significantly as concentration of cocultured TD-MSCs increased. Additionally, mRNA expression of caspase 3 was upregulated with increased concentration of TD-MSCs.
CONCLUSION
TD-MSCs have a potential growth inhibitory effect on HNSCC cell lines in vitro by inducing apoptotic cell death and G1 phase arrest of cell lines.
MeSH Terms
Figure
Reference
-
1. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001; 5. 105(3):369–377. PMID: 11348593.
Article2. Hamada H, Kobune M, Nakamura K, Kawano Y, Kato K, Honmou O, et al. Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Sci. 2005; 3. 96(3):149–156. PMID: 15771617.
Article3. Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006; 5. 203(5):1235–1247. PMID: 16636132.
Article4. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 4. 284(5411):143–147. PMID: 10102814.
Article5. Sasser AK, Mundy BL, Smith KM, Studebaker AW, Axel AE, Haidet AM, et al. Human bone marrow stromal cells enhance breast cancer cell growth rates in a cell line-dependent manner when evaluated in 3D tumor environments. Cancer Lett. 2007; 9. 254(2):255–264. PMID: 17467167.
Article6. Elzaouk L, Moelling K, Pavlovic J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp Dermatol. 2006; 11. 15(11):865–874. PMID: 17002683.
Article7. Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007; 2. 21(2):304–310. PMID: 17170725.
Article8. Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006; 6. 80(3):267–274. PMID: 16214129.
Article10. Dennis JE, Caplan AI. Differentiation potential of conditionally immortalized mesenchymal progenitor cells from adult marrow of a H-2Kb-tsA58 transgenic mouse. J Cell Physiol. 1996; 6. 167(3):523–538. PMID: 8655606.11. Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987; 5. 20(3):263–272. PMID: 3690622.
Article12. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow: analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 3. 6(2):230–247. PMID: 5654088.14. Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995; 12. 18(12):1417–1426. PMID: 7477065.
Article15. Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998; 7. 16(4):406–413. PMID: 9747780.
Article16. Rubinstein P, Rosenfield RE, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood. 1993; 4. 81(7):1679–1690. PMID: 8096404.
Article17. Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002; 9. 20(5):1060–1069. PMID: 12382974.18. Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J Bone Miner Res. 2004; 2. 19(2):256–264. PMID: 14969395.
Article19. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001; 4. 7(2):211–228. PMID: 11304456.
Article20. De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001; 8. 44(8):1928–1942. PMID: 11508446.
Article21. Djouad F, Bony C, Haupl T, Uze G, Lahlou N, Louis-Plence P, et al. Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal stem cells. Arthritis Res Ther. 2005; 7(6):R1304–R1315. PMID: 16277684.22. Shih DT, Lee DC, Chen SC, Tsai RY, Huang CT, Tsai CC, et al. Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells. 2005; 8. 23(7):1012–1020. PMID: 15941858.
Article23. Rzhaninova AA, Gornostaeva SN, Goldshtein DV. Isolation and phenotypical characterization of mesenchymal stem cells from human fetal thymus. Bull Exp Biol Med. 2005; 1. 139(1):134–140. PMID: 16142296.
Article24. Ivanovski S, Gronthos S, Shi S, Bartold PM. Stem cells in the periodontal ligament. Oral Dis. 2006; 7. 12(4):358–363. PMID: 16792719.
Article25. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004; 3. 103(5):1669–1675. PMID: 14576065.
Article26. Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005; 2. 23(2):220–229. PMID: 15671145.
Article27. Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, et al. Isolation of multipotent cells from human term placenta. Stem Cells. 2005; 23(1):3–9. PMID: 15625118.
Article28. Ku JL, Kim WH, Lee JH, Park HS, Kim KH, Sung MW, et al. Establishment and characterization of human laryngeal squamous cell carcinoma cell lines. Laryngoscope. 1999; 6. 109(6):976–982. PMID: 10369293.
Article29. Lee BJ, Lee BH, Wang SG, Lee JC, Roh HJ, Goh EK, et al. Change of the expression of human telomerase reverse transcriptase mRNA and human telomerase RNA after cisplatin and 5-fluorouracil exposure in head and neck squamous cell carcinoma cell lines. J Korean Med Sci. 2007; 9. 22(Suppl):S73–S78. PMID: 17923759.
Article30. Ehtesham M, Stevenson CB, Thompson RC. Stem cell therapies for malignant glioma. Neurosurg Focus. 2005; 9. 19(3):E5. PMID: 16190604.
Article31. Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007; 5. 25(5):1204–1212. PMID: 17218400.
Article32. Mapara KY, Stevenson CB, Thompson RC, Ehtesham M. Stem cells as vehicles for the treatment of brain cancer. Neurosurg Clin N Am. 2007; 1. 18(1):71–80. PMID: 17244555.
Article33. Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005; 4. 65(8):3307–3318. PMID: 15833864.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Adenosquamous Carcinoma Arising from the Tonsil
- p53 expression in squamous cell carcinomas of tongue and tonsil
- A Case of a Primary Branchiogenic Carcinoma with a Synchronous Tonsil Squamous Cell Carcinoma
- Cancer Stem Cells in Head and Neck Cancer
- Uterine Leiomyosarcoma Metastatic to the Palatine Tonsil: A Case Report