J Periodontal Implant Sci.
2011 Dec;41(6):293-301. 10.5051/jpis.2011.41.6.293.
Comparative study on the cellular activities of osteoblast-like cells and new bone formation of anorganic bone mineral coated with tetra-cell adhesion molecules and synthetic cell binding peptide
- Affiliations
-
- 1Department of Periodontology, Kyungpook National University School of Dentistry, Daegu, Korea. jysuh@knu.ac.kr
- 2Institute for Hard Tissue and Bio-Tooth Regeneration, Kyungpook National University School of Dentistry, Daegu, Korea.
- 3Megagen Implant, Gyeongsan, Korea.
- KMID: 2094726
- DOI: http://doi.org/10.5051/jpis.2011.41.6.293
Abstract
- PURPOSE
We have previously reported that tetra-cell adhesion molecule (T-CAM) markedly enhanced the differentiation of osteoblast-like cells grown on anorganic bone mineral (ABM). T-CAM comprises recombinant peptides containing the Arg-Gly-Asp (RGD) sequence in the tenth type III domain, Pro-His-Ser-Arg-Asn (PHSRN) sequence in the ninth type III domain of fibronectin (FN), and the Glu-Pro-Asp-Ilu-Met (EPDIM) and Tyr-His (YH) sequence in the fourth fas-1 domain of betaig-h3. Therefore, the purpose of this study was to evaluate the cellular activity of osteoblast-like cells and the new bone formation on ABM coated with T-CAM, while comparing the results with those of synthetic cell binding peptide (PepGen P-15).
METHODS
To analyze the cell viability, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed, andto analyze gene expression, northernblot was performed. Mineral nodule formations were evaluated using alizarin red stain. The new bone formations of each group were evaluated using histologic observation and histomorphometrc analysis.
RESULTS
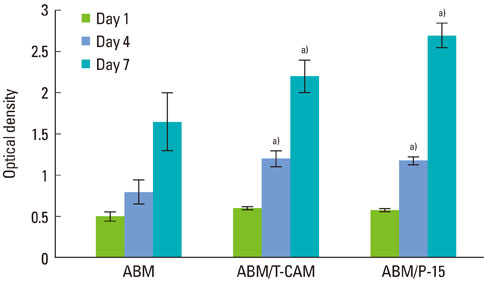
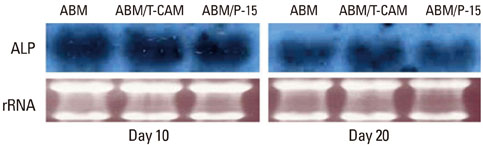
Expression of alkaline phosphatase mRNA was similar in all groups on days 10 and 20. The highest expression of osteopontin mRNA was observed in the group cultured with ABM/P-15, followed by those with ABM/T-CAM and ABM on days 20 and 30. Little difference was seen in the level of expression of collagen type I mRNA on the ABM, ABM/T-CAM, and ABM/P-15 cultured on day 20. There were similar growth and proliferation patterns for the ABM/T-CAM and ABM/P-15. The halo of red stain consistent with Ca2+ deposition was wider and denser around ABM/T-CAM and ABM/P-15 particles than around the ABM particles. The ABM/T-CAM group seemed to have bone forming bioactivity similar to that of ABM/P-15. A complete bony bridge was seen in two thirds of the defects in the ABM/T-CAM and ABM/P-15 groups.
CONCLUSIONS
ABM/T-CAM, which seemed to have bone forming bioactivity similar to ABM/P-15, was considered to serve as effective tissue-engineered bone graft material.
MeSH Terms
-
Alkaline Phosphatase
Anthraquinones
Artificial Cells
Bone Substitutes
Cell Adhesion Molecules
Cell Survival
Collagen Type I
Fibronectins
Gene Expression
Oligopeptides
Osteogenesis
Osteopontin
Peptide Fragments
Peptides
RNA, Messenger
Tetrazolium Salts
Thiazoles
Transplants
Alkaline Phosphatase
Anthraquinones
Bone Substitutes
Cell Adhesion Molecules
Collagen Type I
Fibronectins
Oligopeptides
Osteopontin
Peptide Fragments
Peptides
RNA, Messenger
Tetrazolium Salts
Thiazoles
Figure
Reference
-
1. Boyne PJ. Ciba Foundation. Induction of bone repair by various bone grafting materials. Hard tissue growth, repair and remineralization. Ciba Foundation Symposium 11. 1973. New York: Elsevier;121–141.2. Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983. 174:28–42.
Article3. Urist MR. Bone: formation by autoinduction. 1965. Clin Orthop Relat Res. 2002. 395:4–10.4. Qian JJ, Bhatnagar RS. Enhanced cell attachment to anorganic bone mineral in the presence of a synthetic peptide related to collagen. J Biomed Mater Res. 1996. 31:545–554.
Article5. Scaria PV, Sorensen KR, Bhatnagar RS. Expression of a reactive molecular perspective within the triple helical region of collagen. 11st American Peptide Symposium. 1989. Albuquerque: American Peptide Society;605–607.6. Bhatnagar RS, Qian JJ, Gough CA. The role in cell binding of a beta-bend within the triple helical region in collagen alpha 1 (I) chain: structural and biological evidence for conformational tautomerism on fiber surface. J Biomol Struct Dyn. 1997. 14:547–560.
Article7. Bhatnagar RS, Qian JJ, Wedrychowska A, Sadeghi M, Wu YM, Smith N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999. 5:53–65.
Article8. Nguyen H, Qian JJ, Bhatnagar RS, Li S. Enhanced cell attachment and osteoblastic activity by P-15 peptide-coated matrix in hydrogels. Biochem Biophys Res Commun. 2003. 311:179–186.
Article9. Barboza EP, de Souza RO, Caúla AL, Neto LG, Caúla Fde O, Duarte ME. Bone regeneration of localized chronic alveolar defects utilizing cell binding peptide associated with anorganic bovine-derived bone mineral: a clinical and histological study. J Periodontol. 2002. 73:1153–1159.
Article10. Tehemar S, Hanes P, Sharawy M. Enhancement of osseointegration of implants placed into extraction sockets of healthy and periodontally diseased teeth by using graft material, an ePTFE membrane, or a combination. Clin Implant Dent Relat Res. 2003. 5:193–211.
Article11. Thorwarth M, Schultze-Mosgau S, Wehrhan F, Kessler P, Srour S, Wiltfang J, et al. Bioactivation of an anorganic bone matrix by P-15 peptide for the promotion of early bone formation. Biomaterials. 2005. 26:5648–5657.
Article12. Park JW, Lee SG, Choi BJ, Suh JY. Effects of a cell adhesion molecule coating on the blasted surface of titanium implants on bone healing in the rabbit femur. Int J Oral Maxillofac Implants. 2007. 22:533–541.13. Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985. 40:191–198.
Article14. Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987. 238:491–497.
Article15. Verrier S, Pallu S, Bareille R, Jonczyk A, Meyer J, Dard M, et al. Function of linear and cyclic RGD-containing peptides in osteoprogenitor cells adhesion process. Biomaterials. 2002. 23:585–596.
Article16. Aota S, Nomizu M, Yamada KM. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994. 269:24756–24761.
Article17. Skonier J, Bennett K, Rothwell V, Kosowski S, Plowman G, Wallace P, et al. Beta ig-h3: a transforming growth factor-beta-responsive gene encoding a secreted protein that inhibits cell attachment in vitro and suppresses the growth of CHO cells in nude mice. DNA Cell Biol. 1994. 13:571–584.
Article18. Kawamoto T, Noshiro M, Shen M, Nakamasu K, Hashimoto K, Kawashima-Ohya Y, et al. Structural and phylogenetic analyses of RGD-CAP/beta ig-h3, a fasciclin-like adhesion protein expressed in chick chondrocytes. Biochim Biophys Acta. 1998. 1395:288–292.
Article19. Kim JE, Kim SJ, Lee BH, Park RW, Kim KS, Kim IS. Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-beta-induced gene, betaig-h3. J Biol Chem. 2000. 275:30907–30915.
Article20. Kim JE, Jeong HW, Nam JO, Lee BH, Choi JY, Park RW, et al. Identification of motifs in the fasciclin domains of the transforming growth factor-beta-induced matrix protein betaig-h3 that interact with the alphavbeta5 integrin. J Biol Chem. 2002. 277:46159–46165.
Article21. Krammer A, Craig D, Thomas WE, Schulten K, Vogel V. A structural model for force regulated integrin binding to fibronectin's RGD-synergy site. Matrix Biol. 2002. 21:139–147.
Article22. Bodine PV, Green J, Harris HA, Bhat RA, Stein GS, Lian JB, et al. Functional properties of a conditionally phenotypic, estrogen-responsive, human osteoblast cell line. J Cell Biochem. 1997. 65:368–387.
Article23. Franceschi RT. The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit Rev Oral Biol Med. 1999. 10:40–57.
Article24. Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992. 69:11–25.
Article25. Clover J, Dodds RA, Gowen M. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 1992. 103(Pt 1):267–271.
Article26. Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res. 1997. 12:1189–1197.
Article27. Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001. 28:174–181.
Article28. Saito T, Albelda SM, Brighton CT. Identification of integrin receptors on cultured human bone cells. J Orthop Res. 1994. 12:384–394.
Article29. Bhatnagar RS, QiannJJ , Anna Wedychowska A, Dixon E, Smith N. Biomimetic habitats for cells: ordered matrix deposition and differentiation in gingival fibroblasts cultured on hydroxyapatitie coated with a collagen analogue. Cell Mater. 1999. 9:93–104.30. Krauser JT, Rohrer MD, Wallace SS. Human histologic and histomorphometric analysis comparing OsteoGraf/N with PepGen P-15 in the maxillary sinus elevation procedure: a case report. Implant Dent. 2000. 9:298–302.
Article31. Kim TI, Jang JH, Chung CP, Ku Y. Fibronectin fragment promotes osteoblast-associated gene expression and biological activity of human osteoblast-like cell. Biotechnol Lett. 2003. 25:2007–2011.
Article32. Choi JY, Lee BH, Song KB, Park RW, Kim IS, Sohn KY, et al. Expression patterns of bone-related proteins during osteoblastic differentiation in MC3T3-E1 cells. J Cell Biochem. 1996. 61:609–618.
Article33. Genge BR, Sauer GR, Wu LN, McLean FM, Wuthier RE. Correlation between loss of alkaline phosphatase activity and accumulation of calcium during matrix vesicle-mediated mineralization. J Biol Chem. 1988. 263:18513–18519.
Article34. Ignatius A, Blessing H, Liedert A, Schmidt C, Neidlinger-Wilke C, Kaspar D, et al. Tissue engineering of bone: effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials. 2005. 26:311–318.
Article35. Turhani D, Weissenböck M, Watzinger E, Yerit K, Cvikl B, Ewers R, et al. In vitro study of adherent mandibular osteoblast-like cells on carrier materials. Int J Oral Maxillofac Surg. 2005. 34:543–550.
Article36. Iseki S, Wilkie AO, Heath JK, Ishimaru T, Eto K, Morriss-Kay GM. Fgfr2 and osteopontin domains in the developing skull vault are mutually exclusive and can be altered by locally applied FGF2. Development. 1997. 124:3375–3384.
Article37. Iseki S, Wilkie AO, Morriss-Kay GM. Fgfr1 and Fgfr2 have distinct differentiation- and proliferation-related roles in the developing mouse skull vault. Development. 1999. 126:5611–5620.
Article38. Park MH, Shin HI, Choi JY, Nam SH, Kim YJ, Kim HJ, et al. Differential expression patterns of Runx2 isoforms in cranial suture morphogenesis. J Bone Miner Res. 2001. 16:885–892.
Article39. Kubota T, Yamauchi M, Onozaki J, Sato S, Suzuki Y, Sodek J. Influence of an intermittent compressive force on matrix protein expression by ROS 17/2.8 cells, with selective stimulation of osteopontin. Arch Oral Biol. 1993. 38:23–30.
Article40. Valentin AH, Weber J. Receptor technology--cell binding to P-15: a new method of regenerating bone quickly and safely-preliminary histomorphometrical and mechanical results in sinus floor augmentations. Keio J Med. 2004. 53:166–171.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with synthetic cell binding peptide sequences
- Effects of H2O2 Derived Hydroxyl Radicals Treated Fibronectin on Rat Calvarial Osteoblast
- The Effects of Dexamethasone on Growth and Differentiation of Osteoblast-like Cell
- The biologic effect of fibrin-binding synthetic oligopeptide on periodontal ligament cells
- Initial adhesion of bone marrow stromal cells to various bone graft substitutes