J Periodontal Implant Sci.
2011 Apr;41(2):67-72.
Initial adhesion of bone marrow stromal cells to various bone graft substitutes
- Affiliations
-
- 1Department of Periodontology, Seoul National University School of Dentistry, Seoul, Korea. icrhyu@snu.ac.kr
Abstract
- PURPOSE
The aim of this study is to determine whether certain biomaterials have the potential to support cell attachment. After seeding bone marrow stromal cells onto the biomaterials, we investigated their responses to each material in vitro.
METHODS
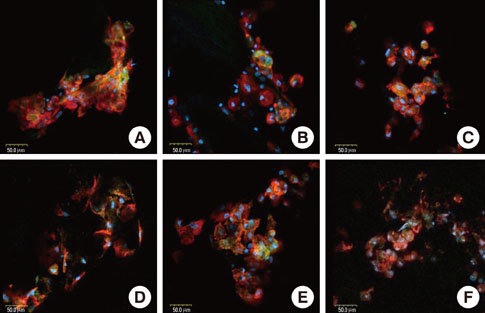
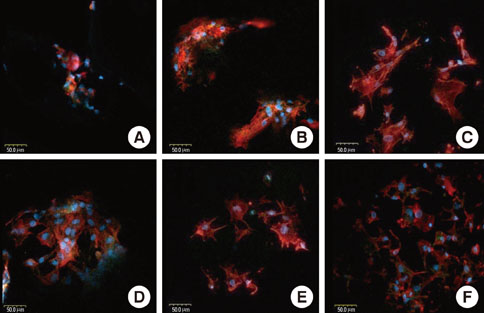
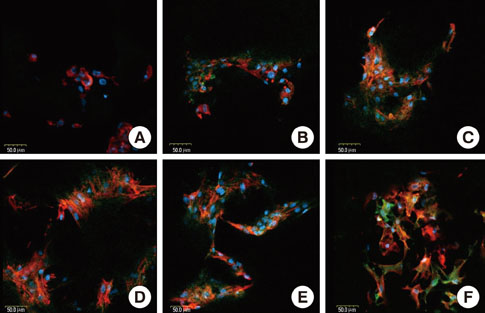
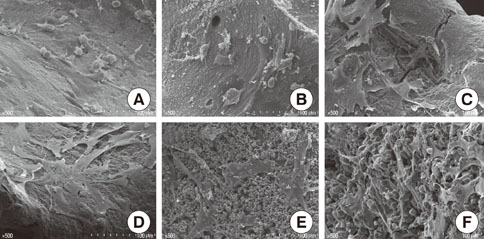
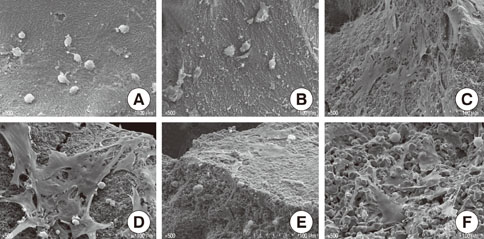
Rat bone marrow derived stromal cells were used. The biomaterials were deproteinized bovine bone mineral (DBBM), DBBM coated with fibronectin (FN), synthetic hydroxyapatite (HA), HA coated with FN, HA coated with beta-tricalcium phosphate (TCP), and pure beta-TCP. With confocal laser scanning microscopy, actin filaments and vinculin were observed after 6, 12, and 24 hours of cell seeding. The morphological features of cells on each biomaterial were observed using scanning electron microscopy at day 1 and 7.
RESULTS
The cells on HA/FN and HA spread widely and showed better defined actin cytoskeletons than those on the other biomaterials. At the initial phase, FN seemed to have a favorable effect on cell adhesion. In DBBM, very few cells adhered to the surface.
CONCLUSIONS
Within the limitations of this study, we can conclude that in contrast with DBBM not supporting cell attachment, HA provided a more favorable environment with respect to cell attachment.
Keyword
MeSH Terms
-
Actin Cytoskeleton
Animals
Biocompatible Materials
Bone Marrow
Bone Substitutes
Calcium Phosphates
Cell Adhesion
Durapatite
Fibronectins
Mesenchymal Stromal Cells
Microscopy, Confocal
Microscopy, Electron, Scanning
Rats
Seeds
Stem Cells
Stromal Cells
Transplants
Vinculin
Biocompatible Materials
Bone Substitutes
Calcium Phosphates
Durapatite
Fibronectins
Vinculin
Figure
Reference
-
1. van den Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PH, Mikos AG, Jansen JA. Effect of fibronectin- and collagen I-coated titanium fiber mesh on proliferation and differentiation of osteogenic cells. Tissue Eng. 2003; 9:505–515.
Article2. Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003; 24:2077–2082.
Article3. Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007; 28:335–343.
Article4. Langer R, Vacanti JP. Tissue engineering. Science. 1993; 260:920–926.
Article5. Turhani D, Weissenböck M, Watzinger E, Yerit K, Cvikl B, Ewers R, et al. Invitro study of adherent mandibular osteoblast-like cells on carrier materials. Int J Oral Maxillofac Surg. 2005; 34:543–550.
Article6. Okumura A, Goto M, Goto T, Yoshinari M, Masuko S, Katsuki T, et al. Substrate affects the initial attachment and subsequent behavior of human osteoblastic cells (Saos-2). Biomaterials. 2001; 22:2263–2271.
Article7. Ohgushi H, Okumura M, Tamai S, Shors EC, Caplan AI. Marrow cell induced osteogenesis in porous hydroxyapatite and tricalcium phosphate: a comparative histomorphometric study of ectopic bone formation. J Biomed Mater Res. 1990; 24:1563–1570.
Article8. Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000; 49:328–337.
Article9. Hattori H, Masuoka K, Sato M, Ishihara M, Asazuma T, Takase B, et al. Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. J Biomed Mater Res B Appl Biomater. 2006; 76:230–239.
Article10. Marino G, Rosso F, Cafiero G, Tortora C, Moraci M, Barbarisi M, et al. Beta-tricalcium phosphate 3D scaffold promote alone osteogenic differentiation of human adipose stem cells: in vitro study. J Mater Sci Mater Med. 2010; 21:353–363.
Article11. Dong J, Uemura T, Shirasaki Y, Tateishi T. Promotion of bone formation using highly pure porous beta-TCP combined with bone marrow-derived osteoprogenitor cells. Biomaterials. 2002; 23:4493–4502.
Article12. Boo JS, Yamada Y, Okazaki Y, Hibino Y, Okada K, Hata K, et al. Tissue-engineered bone using mesenchymal stem cells and a biodegradable scaffold. J Craniofac Surg. 2002; 13:231–239.
Article13. Camelo M, Nevins ML, Schenk RK, Simion M, Rasperini G, Lynch SE, et al. Clinical, radiographic, and histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Gide. Int J Periodontics Restorative Dent. 1998; 18:321–331.14. Stephan EB, Jiang D, Lynch S, Bush P, Dziak R. Anorganic bovine bone supports osteoblastic cell attachment and proliferation. J Periodontol. 1999; 70:364–369.
Article15. Piattelli M, Favero GA, Scarano A, Orsini G, Piattelli A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: a histologic long-term report of 20 cases in humans. Int J Oral Maxillofac Implants. 1999; 14:835–840.16. Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J Bone Miner Res. 1994; 9:487–496.
Article17. Grzesik WJ, Ivanov B, Robey FA, Southerland J, Yamauchi M. Synthetic integrin-binding peptides promote adhesion and proliferation of human periodontal ligament cells in vitro. J Dent Res. 1998; 77:1606–1612.
Article18. Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988; 254:317–330.
Article19. Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000; 21:667–681.
Article20. Brodie JC, Goldie E, Connel G, Merry J, Grant MH. Osteoblast interactions with calcium phosphate ceramics modified by coating with type I collagen. J Biomed Mater Res A. 2005; 73:409–421.
Article21. Petrovic L, Schlegel AK, Schultze-Mosgau S, Wiltfang J. Different substitute biomaterials as potential scaffolds in tissue engineering. Int J Oral Maxillofac Implants. 2006; 21:225–231.22. Herten M, Rothamel D, Schwarz F, Friesen K, Koegler G, Becker J. Surface- and nonsurface-dependent in vitro effects of bone substitutes on cell viability. Clin Oral Investig. 2009; 13:149–155.
Article23. Wiedmann-Al-Ahmad M, Gutwald R, Gellrich NC, Hübner U, Schmelzeisen R. Search for ideal biomaterials to cultivate human osteoblast-like cells for reconstructive surgery. J Mater Sci Mater Med. 2005; 16:57–66.
Article24. Kübler A, Neugebauer J, Oh JH, Scheer M, Zöller JE. Growth and proliferation of human osteoblasts on different bone graft substitutes: an in vitro study. Implant Dent. 2004; 13:171–179.
Article25. Açil Y, Terheyden H, Dunsche A, Fleiner B, Jepsen S. Three-dimensional cultivation of human osteoblast-like cells on highly porous natural bone mineral. J Biomed Mater Res. 2000; 51:703–710.
Article26. Ignjatović N, Ninkov P, Kojić V, Bokurov M, Srdić V, Krnojelac D, et al. Cytotoxicity and fibroblast properties during in vitro test of biphasic calcium phosphate/poly-dl-lactide-co-glycolide biocomposites and different phosphate materials. Microsc Res Tech. 2006; 69:976–982.
Article27. Carmagnola D, Adriaens P, Berglundh T. Healing of human extraction sockets filled with Bio-Oss. Clin Oral Implants Res. 2003; 14:137–143.28. Zitzmann NU, Schärer P, Marinello CP, Schüpbach P, Berglundh T. Alveolar ridge augmentation with Bio-Oss: a histologic study in humans. Int J Periodontics Restorative Dent. 2001; 21:288–295.29. Burmeister JS, Vrany JD, Reichert WM, Truskey GA. Effect of fibronectin amount and conformation on the strength of endothelial cell adhesion to HEMA/EMA copolymers. J Biomed Mater Res. 1996; 30:13–22.
Article30. Suzuki T, Hukkanen M, Ohashi R, Yokogawa Y, Nishizawa K, Nagata F, et al. Growth and adhesion of osteoblast-like cells derived from neonatal rat calvaria on calcium phosphate ceramics. J Biosci Bioeng. 2000; 89:18–26.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Human Bone Marrow Stromal Cells with Fibroblasts in Cell Proliferation and Collagen Synthesis
- An Immune-compromised Method for Tooth Transplantation Using Adult Bone Marrow Stromal Cells and Embryonic Tooth Germ
- Bone Substitutes: From Basic to Current Update
- Comparison of Bone Marrow Stromal Cells with Fibroblasts in Wound Healing Accelerating Growth Factor Secretion
- Effective Preparation of A Bone Marrow Graft