Korean Circ J.
2007 Sep;37(9):399-407. 10.4070/kcj.2007.37.9.399.
Arrhythmogenic Gene Change and Nerve Sprouting after Acute Myocardial Infarction in Mice
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, The Catholic University of Korea, Seoul, Korea. oys@catholic.ac.kr
- 2Cedars-Sinai Medical Center David Geffen School of Medicine, UCLA, Los Angeles, CA (MCF), USA.
- KMID: 2093975
- DOI: http://doi.org/10.4070/kcj.2007.37.9.399
Abstract
-
BACKGROUND AND OBJECTIVES: Myocardial infarction (MI) elicits nerve sprouting. However, the time course and spatial distribution of this nerve sprouting and its relationship to the expression of neurotrophic factors is unclear. The aim of this study was to identify the association of nerve sprouting with the expression of neurotrophic factors.
MATERIALS AND METHODS
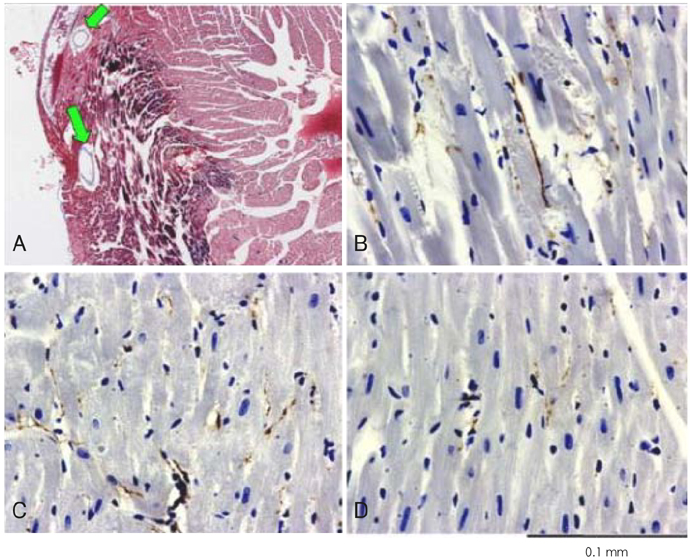
We induced MI in FVB mice by ligating the left coronary artery. The hearts were removed at 3 hours to 13 months after MI for growth associated protein 43 (GAP-43) immunostaining. The nerve density (micrometer2/mm2) was determined by ImagePro software. In another group of mice, their myocardial tissues were processed and analyzed with using an Affymetrix RG U74V2 array.
RESULTS
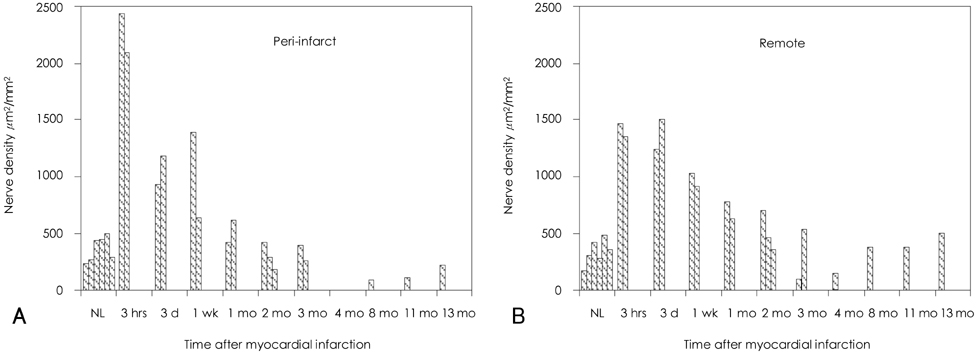
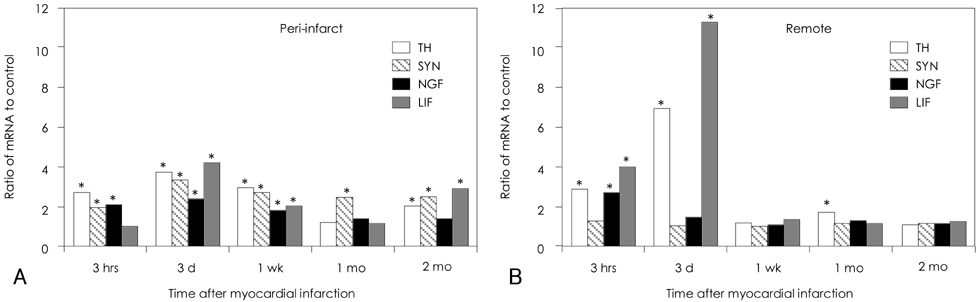
The density of the nerve fibers that were immunopositive for GAP-43 was the highest 3 hours after MI in both the peri-infarct areas and the remote areas. The outer loop of the ventricle had a higher nerve density than that in the inner loop of the ventricle. The differences were at a peak 3 hours after MI, but they persisted for 2 months afterwards. The expressions of nerve growth factor, insulin-like growth factor, leukemia inhibitory factor, transforming growth factor-beta3 and interleukin-1alpha were increased for up to 2 months after MI as compared to the normal control. qRT PCR analyses showed increased mRNA for tyrosine hydroxylase, synaptophysin, nerve growth factor and leukemia inhibiting factor in the peri-infarct areas for up to 2 months after MI, but this occurred only for roughly 3 days after MI in the remote areas.
CONCLUSION
We conclude that MI resulted in immediate upregulation of nerve growth factor, insulin-like growth factor, leukemia inhibitory factor, transforming growth factor-beta3 and interleukin-1alpha in the peri-infarct areas and this all occurred to a lesser extent in the remote areas. These changes persisted for at least 2 months, and they were associated with increased nerve sprouting activity, which was most active in the outer loop of the heart.
Keyword
MeSH Terms
-
Animals
Coronary Vessels
DNA
Electrophysiology
GAP-43 Protein
Heart
Interleukin-1alpha
Leukemia
Leukemia Inhibitory Factor
Mice*
Myocardial Infarction*
Nerve Fibers
Nerve Growth Factor
Nerve Growth Factors
Polymerase Chain Reaction
Regeneration
RNA, Messenger
Synaptophysin
Tyrosine 3-Monooxygenase
Up-Regulation
Ventricular Remodeling
DNA
GAP-43 Protein
Interleukin-1alpha
Leukemia Inhibitory Factor
Nerve Growth Factor
Nerve Growth Factors
RNA, Messenger
Synaptophysin
Tyrosine 3-Monooxygenase
Figure
Reference
-
1. Guth L. Regeneration in the mammalian peripheral nervous system. Physiol Rev. 1956. 36:441–478.2. Levi-Montalcini R. Growth control of nerve cells by a protein factor and its antiserum. Science. 1964. 143:105–110.3. Hirsch EF, Jellinik M, Cooper T, Kaiser GC, Barner HC, Borghard-Erdle AM. The Innervation of the Vertebrate Heart. 1970. Springfield:4. Barber MJ, Mueller TM, Davies BG, Gill RM, Zipes DP. Interruption of sympathetic and vagal-mediated afferent responses by transmural myocardial infarction. Circulation. 1985. 72:623–631.5. Nori SL, Gaudino M, Alessandrini F, Bronzetti E, Santarelli P. Immunohistochemical evidence for sympathetic denervation and reinnervation after necrotic injury in rat myocardium. Cell Mol Biol. 1995. 41:799–807.6. Vracko R, Thorning D, Frederickson RG. Fate of nerve fibers in necrotic, healing, and healed rat myocardium. Lab Invest. 1990. 63:490–501.7. Vracko R, Thorning D, Frederickson RG. Nerve fibers in human myocardial scars. Hum Pathol. 1991. 22:138–146.8. Cao JM, Fishbein MC, Han JB, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000. 101:1960–1969.9. Zhou S, Chen LS, Miyauchi Y, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004. 95:76–83.10. Torrent-Guasp F, Buckberg GD, Clemente C, Cox JL, Coghlan HC, Gharib M. The structure and function of the helical heart and its buttress wrapping: I. the normal macroscopic structure of the heart. Semin Thorac Cardiovasc Surg. 2001. 13:301–319.11. Buckberg GD, Weisfeldt ML, Ballester M, et al. Left ventricular form and function: scientific priorities and strategic planning for development of new views of disease. Circulation. 2004. 110:e333–e336.12. Cao JM, Chen LS, KenKnight BH, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000. 86:816–821.13. Zhou S, Cao JM, Tebb ZD, et al. Modulation of QT interval by cardiac sympathetic nerve sprouting and the mechanisms of ventricular arrhythmia in a canine model of sudden cardiac death. J Cardiovasc Electrophysiol. 2001. 12:1068–1073.14. Abe T, Morgan DA, Gutterman DD. Protective role of nerve growth factor against postischemic dysfunction of sympathetic coronary innervation. Circulation. 1997. 95:213–220.15. Fuchs M, Hilfiker A, Kaminski K, et al. Role of interleukin-6 for LV remodeling and survival after experimental myocardial infarction. FASEB J. 2003. 17:2118–2120.16. Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004. 94:1543–1553.17. Gwechenberger M, Mendoza LH, Youker KA, et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999. 99:546–551.18. Reiss K, Meggs LG, Li P, Olivetti G, Capasso JM, Anversa P. Upregulation of IGF1, IGF1-receptor, and late growth related genes in ventricular myocytes acutely after infarction in rats. J Cell Physiol. 1994. 158:160–168.19. Conti E, Andreotti F, Sciahbasi A, et al. Markedly reduced insulin-like growth factor-1 in the acute phase of myocardial infarction. J Am Coll Cardiol. 2001. 38:26–32.20. Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta (1). Mol Genet Metab. 2000. 71:418–435.21. Drapeau J, El Helou V, Clement R, et al. Nestin-expressing neural stem cells identified in the scar following myocardial infarction. J Cell Physiol. 2005. 204:51–62.22. Park JW, Youn HJ, Chung WS, et al. Changes of responses of autonomic nervous system in patients after myocardial infarction. Korean Circ J. 1994. 24:272–279.23. Lee TI, Choi KW, Kim YJ, et al. Effect of stress on cardiovascular autonomic nervouse activity in recovering myocardial infarction patients and normal controls measured by power spectral analysis. Korean Circ J. 1994. 24:24–37.24. Zou Y, Takano H, Mizukami M, et al. Leukemia inhibitory factor enhances survival of cardiomyocytes and induces regeneration of myocardium after myocardial infarction. Circulation. 2003. 108:748–753.25. Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM. Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci U S A. 1995. 92:8031–8035.26. Kotlyar AA, Vered Z, Goldberg I, et al. Insulin-like growth factor I and II preserve myocardial structure in postinfarct swine. Heart. 2001. 86:693–700.27. Kim DT, Luthringer DJ, Lai AC, et al. Sympathetic nerve sprouting after orthotopic heart transplantation. J Heart Lung Transplant. 2004. 23:1349–1358.28. Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Myocardial efficiency and sympathetic reinnervation after orthotopic heart transplantation: a noninvasive study with positron emission tomography. Circulation. 2001. 103:1881–1886.29. Luisi AJ, Fallavollita JA, Suzuki G, Canty JM. Spatial inhomogeneity of sympathetic nerve function in hibernating myocardium. Circulation. 2002. 106:779–781.30. Canty JM, Suzuki G, Banas MD, Verheyen F, Borgers M, Fallavollita JA. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res. 2004. 94:1142–1149.31. Cho IH, Lee JY, Shin DG, et al. Influence of autonomic nervous system in occlusion and reperfusion arrhythmia. Korean Circ J. 1990. 20:369–380.32. Zolotareva AG, Kogan ME. Production of experimental occlusive myocardial infarction in mice. Cor Vasa. 1978. 20:308–314.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Invasive Treatment of Acute Myocardial Infarction: What is the Optimal Therapy for Acute Myocardial Infarction?
- Primary Coronary Stenting as a Successful Treatment of Acute Myocardial
- Angiotensin-converting enzyme gene polymorphism is not associated with myocardial infarction in Koreans
- Left Side Otalgia Caused by Acute Myocardial Infarction
- Serum Myoglobin in the Early Phase of Acute Myocardial Infarction