Electrolyte Blood Press.
2008 Jun;6(1):22-26. 10.5049/EBP.2008.6.1.22.
The Role of Angiopoietin-1 in Kidney Disease
- Affiliations
-
- 1 Department of Internal Medicine, Chonbuk National University Medical School, Jeonju, Korea. kwon@chonbuk.ac.kr
- KMID: 2052302
- DOI: http://doi.org/10.5049/EBP.2008.6.1.22
Abstract
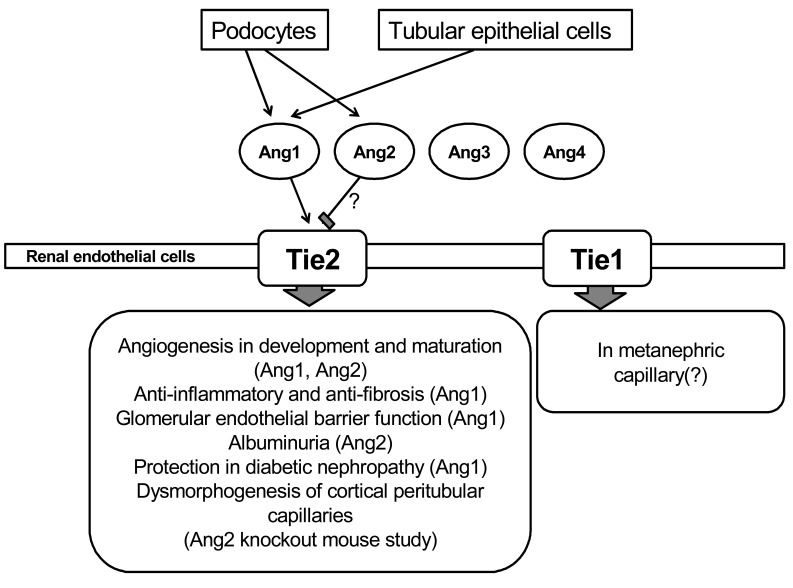
- Injury to the renal microvasculature and inflammatory process may be major factors in the progression of renal disease, therefore, protection of the renal endothelial cell and regulation of inflammatory process may be an important therapeutic target of renal disease. Thus, we evaluated the protective effect of cartilage oligomeric matrix protein-angiopoietin-1 (COMP-Ang1) in unilateral ureteral obstruction (UUO)-induced renal fibrosis, cyclosporine A (CsA)-induced renal injury, and the diabetic nephropathy model. In the UUO model, morphologic examination indicated less tubular injury and tubulointerstitial fibrosis in mice that received COMP-Ang1 compared to vehicle-treated mice. Interstitial type I collagen, myofibroblast accumulation, renal surface microvasculature and renal blood flow were higher after treatment with COMP-Ang1 compared to vehicle-treated mice. COMP-Ang1 treatment decreased monocyte/macrophage infiltration, tissue levels of transforming growth factor beta1, and Smad 2/3 phosphorylation and increased Smad 7 in the obstructed kidney. In CsA-induced renal injury, histologic examination showed significantly decreased CsA- induced tubular damage and tubulointerstitial fibrosis in COMP-Ang1 treated mice. COMP-Ang1 administration also decreased increased macrophage infiltration, adhesion molecule expression, TGF-beta1, and Smad 2/3 levels in CsA-treated kidneys, while increasing Smad 7 levels. Laser-Doppler sonographic findings and endothelial factor VIII staining revealed that COMP-Ang1 had a preservative effect on peritubular vasculature. In the diabetic nephropathy model, COMP-Ang1 reduced albuminuria and decreased mesangial expansion, thickening of the glomerular basement membrane and podocyte foot process broadening and effacement. COMP-Ang1 may delay the fibrotic changes in the kidney of diabetic db/db mice through its anti- inflammatory or metabolic effects. In conclusion, COMP-Ang1 may be an endothelium-specific and anti- inflammatory therapeutic modality in fibrotic renal disease.
MeSH Terms
-
Albuminuria
Angiopoietin-1
Animals
Cartilage
Collagen Type I
Cyclosporine
Diabetic Nephropathies
Endothelial Cells
Factor VIII
Fibrosis
Foot
Glomerular Basement Membrane
Kidney
Kidney Diseases
Macrophages
Mice
Microvessels
Myofibroblasts
Phosphorylation
Podocytes
Renal Circulation
Transforming Growth Factor beta1
Ureteral Obstruction
Angiopoietin-1
Collagen Type I
Cyclosporine
Factor VIII
Transforming Growth Factor beta1
Figure
Reference
-
1. Davis S, Yancopoulos GD. The angiopoietins: Yin and Yang in angiogenesis. Curr Top Microbiol Immunol. 1999; 237:173–185. PMID: 9893351.
Article2. Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci U S A. 1999; 96:1904–1909. PMID: 10051567.
Article3. Nishimura M, Miki T, Yashima R, Yokoi N, Yano H, Sato Y, et al. Angiopoietin-3, a novel member of the angiopoietin family. FEBS Lett. 1999; 448:254–256. PMID: 10218486.
Article4. Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998; 8:529–532. PMID: 9560344.
Article5. Kukk E, Wartiovaara U, Gunji Y, Kaukonen J, Buhring HJ, Rappold I, et al. Analysis of Tie receptor tyrosine kinase in haemopoietic progenitor and leukaemia cells. Br J Haematol. 1997; 98:195–203. PMID: 9233584.
Article6. Suda T. Function of TIE2/angiopoietin in the angiogenesis and hematopoiesis. Rinsho Ketsueki. 2000; 41:251–255. PMID: 10846448.7. Schnurch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993; 119:957–968. PMID: 8187650.
Article8. Breier G, Damert A, Plate KH, Risau W. Angiogenesis in embryos and ischemic diseases. Thromb Haemost. 1997; 78:678–683. PMID: 9198238.
Article9. Breier G. Angiogenesis in embryonic development--a review. Placenta. 2000; 21(Suppl A):S11–S15. PMID: 10831116.
Article10. Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999; 448:249–253. PMID: 10218485.
Article11. Kwak HJ, Lee SJ, Lee YH, Ryu CH, Koh KN, Choi HY, et al. Angiopoietin-1 inhibits irradiation- and mannitol-induced apoptosis in endothelial cells. Circulation. 2000; 101:2317–2324. PMID: 10811601.12. Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3'-Kinase/Akt signal transduction pathway. Circ Res. 2000; 86:24–29. PMID: 10625301.
Article13. Cho CH, Kammerer RA, Lee HJ, Yasunaga K, Kim KT, Choi HH, et al. Designed angiopoietin-1 variant, COMP-Ang1, protects against radiation-induced endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004; 101:5553–5558. PMID: 15060280.
Article14. Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, et al. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000; 86:952–959. PMID: 10807867.
Article15. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997; 277:55–60. PMID: 9204896.
Article16. Marti HH, Risau W. Angiogenesis in ischemic disease. Thromb Haemost. 1999; 82(Suppl 1):44–52. PMID: 10695485.
Article18. Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999; 286:2511–2514. PMID: 10617467.
Article19. Fujikawa K, de Aos Scherpenseel I, Jain SK, Presman E, Christensen RA, Varticovski L. Role of PI 3-kinase in angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells. Exp Cell Res. 1999; 253:663–672. PMID: 10585289.
Article20. Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000; 6:460–463. PMID: 10742156.
Article21. Satchell SC, Mathieson PW. Angiopoietins: microvascular modulators with potential roles in glomerular pathophysiology. J Nephrol. 2003; 16:168–178. PMID: 12768063.22. Yuan HT, Suri C, Yancopoulos GD, Woolf AS. Expression of angiopoietin-1, angiopoietin-2, and the Tie-2 receptor tyrosine kinase during mouse kidney maturation. J Am Soc Nephrol. 1999; 10:1722–1736. PMID: 10446940.
Article23. Woolf AS, Yuan HT. Angiopoietin growth factors and Tie receptor tyrosine kinases in renal vascular development. Pediatr Nephrol. 2001; 16:177–184. PMID: 11261688.
Article24. Satchell SC, Harper SJ, Tooke JE, Kerjaschki D, Saleem MA, Mathieson PW. Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor. J Am Soc Nephrol. 2002; 13:544–550. PMID: 11805186.
Article25. Lu YH, Deng AG, Li N, Song MN, Yang X, Liu JS. Changes in angiopoietin expression in glomeruli involved in glomerulosclerosis in rats with daunorubicin-induced nephrosis. Acta Pharmacol Sin. 2006; 27:579–587. PMID: 16626513.26. Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004; 15:566–574. PMID: 14978158.
Article27. Long DA, Woolf AS, Suda T, Yuan HT. Increased renal angiopoietin-1 expression in folic acid-induced nephrotoxicity in mice. J Am Soc Nephrol. 2001; 12:2721–2731. PMID: 11729241.
Article28. Yuan HT, Tipping PG, Li XZ, Long DA, Woolf AS. Angiopoietin correlates with glomerular capillary loss in anti-glomerular basement membrane glomerulonephritis. Kidney Int. 2002; 61:2078–2089. PMID: 12028448.
Article29. Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992; 20:1–17. PMID: 1621674.
Article30. Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968; 2:363–366. PMID: 4173786.
Article31. Bohle A, Mackensen-Haen S, Wehrmann M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res. 1996; 19:191–195. PMID: 8887259.
Article32. Ohashi R, Kitamura H, Yamanaka N. Peritubular capillary injury during the progression of experimental glomerulonephritis in rats. J Am Soc Nephrol. 2000; 11:47–56. PMID: 10616839.
Article33. Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001; 12:1434–1447. PMID: 11423572.
Article34. Ohashi R, Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, et al. Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol. 2002; 13:1795–1805. PMID: 12089375.
Article35. Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol. 2002; 13:96–107. PMID: 11752026.
Article36. Morrissey J, Hruska K, Guo G, Wang S, Chen Q, Klahr S. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol. 2002; 13(Suppl 1):S14–S21. PMID: 11792757.
Article37. Chevalier RL, Klahr S. Therapeutic approaches in obstructive uropathy. Semin Nephrol. 1998; 18:652–658. PMID: 9819156.38. Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, et al. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci U S A. 2004; 101:5547–5552. PMID: 15060279.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Study on the Expression of Angiopoietin-1, Angiopoietin-2, and Tie2 in Mouse Kidney Maturation
- Hypertension, Vascular Rarefaction and Angiopoietin-1
- Expression of Angiopoietin-1 and Angiopoietin-2 mRNA in Eutopic and Ectopic Endometrium
- Midtrimester maternal plasma concentrations of angiopoietin 1, angiopoietin 2, and placental growth factor in pregnant women who subsequently develop preeclampsia
- Angiopoietins in Diabetic Nephropathy