Clin Exp Vaccine Res.
2015 Jan;4(1):83-87. 10.7774/cevr.2015.4.1.83.
Comparative evaluation of booster efficacies of BCG, Ag85B, and Ag85B peptides based vaccines to boost BCG induced immunity in BALB/c mice: a pilot study
- Affiliations
-
- 1Biochemistry Research Laboratory, Central India Institute of Medical Sciences, Nagpur, India. raj_ciims@rediffmail.com
- 2Department of Veterinary Microbiology and Animal Biotechnology, Nagpur Veterinary College, Nagpur, India.
- KMID: 2049111
- DOI: http://doi.org/10.7774/cevr.2015.4.1.83
Abstract
- PURPOSE
In the present study booster efficacies of Ag85 B, Bacillus Calmette-Guerin (BCG), and Ag85B peptides were evaluated using prime boost regimes in BALB/c mice.
MATERIALS AND METHODS
Mice were primed with BCG vaccine and subsequently boosted with Ag85B, BCG and cocktail of Ag85B peptides.
RESULTS
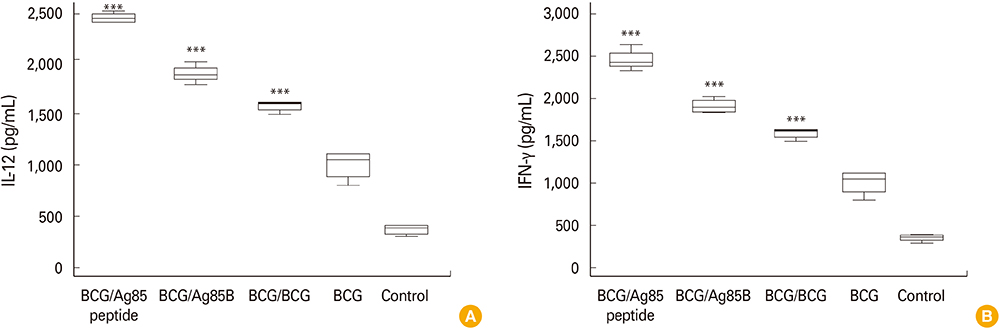
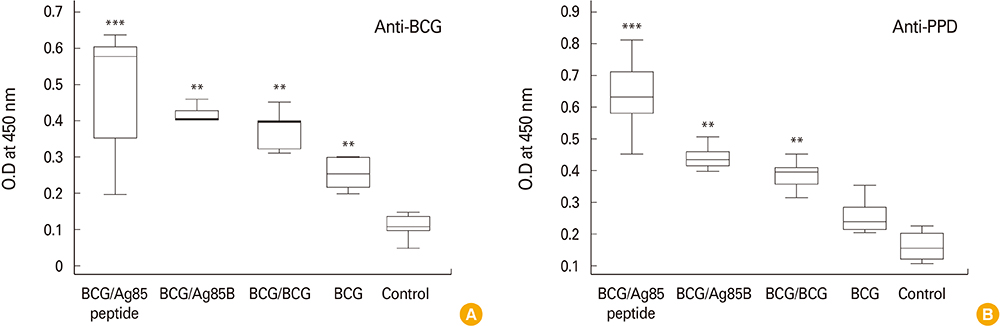
Based on analysis of immune response it was observed mice boosted with Ag85B peptides showed significant (p < 0.001) cytokines levels (interferon gamma, interleukin 12) and BCG specific antibodies (anti-BCG and anti-purified protein derivative titre) compared to booster dose of BCG, Ag85B and BCG alone.
CONCLUSION
Our pilot results suggest that prime boost regimes with Ag85B peptides can boost waning BCG induced immunity and may improve immunogenicity of BCG vaccine. However, lot of work is further needed using experimental model of tuberculosis infection to justify the result.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Assessment of immunological markers and booster effects of Ag85B peptides, Ag85B, and BCG in blood of BCG vaccinated children: a preliminary report
Aliabbas A. Husain, Hatim F. Daginawla, Lokendra Singh, Rajpal S. Kashyap
Clin Exp Vaccine Res. 2016;5(1):31-40. doi: 10.7774/cevr.2016.5.1.31.
Reference
-
1. Gupta UD, Katoch VM. Animal models of tuberculosis for vaccine development. Indian J Med Res. 2009; 129:11–18.2. Gupta UD, Katoch VM, McMurray DN. Current status of TB vaccines. Vaccine. 2007; 25:3742–3751.
Article3. Agger EM, Andersen P. A novel TB vaccine; towards a strategy based on our understanding of BCG failure. Vaccine. 2002; 21:7–14.
Article4. Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013; 381:1021–1028.
Article5. Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006; 4:469–476.
Article6. Allen PM, Strydom DJ, Unanue ER. Processing of lysozyme by macrophages: identification of the determinant recognized by two T-cell hybridomas. Proc Natl Acad Sci U S A. 1984; 81:2489–2493.
Article7. Husain AA, Kashyap RS, Kalorey DR, et al. Effect of repeat dose of BCG vaccination on humoral response in mice model. Indian J Exp Biol. 2011; 49:7–10.8. Mustafa AS, Shaban FA, Abal AT, et al. Identification and HLA restriction of naturally derived Th1-cell epitopes from the secreted Mycobacterium tuberculosis antigen 85B recognized by antigen-specific human CD4(+) T-cell lines. Infect Immun. 2000; 68:3933–3940.
Article9. Valle MT, Megiovanni AM, Merlo A, et al. Epitope focus, clonal composition and Th1 phenotype of the human CD4 response to the secretory mycobacterial antigen Ag85. Clin Exp Immunol. 2001; 123:226–232.
Article10. Kashyap RS, Shekhawat SD, Nayak AR, Purohit HJ, Taori GM, Daginawala HF. Diagnosis of tuberculosis infection based on synthetic peptides from Mycobacterium tuberculosis antigen 85 complex. Clin Neurol Neurosurg. 2013; 115:678–683.
Article11. Barreto ML, Pereira SM, Ferreira AA. BCG vaccine: efficacy and indications for vaccination and revaccination. J Pediatr (Rio J). 2006; 82:3 Suppl. S45–S54.
Article12. McShane H, Hill A. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 2005; 7:962–967.
Article13. Martin C. The dream of a vaccine against tuberculosis; new vaccines improving or replacing BCG? Eur Respir J. 2005; 26:162–167.
Article14. Olsen AW, Hansen PR, Holm A, Andersen P. Efficient protection against Mycobacterium tuberculosis by vaccination with a single subdominant epitope from the ESAT-6 antigen. Eur J Immunol. 2000; 30:1724–1732.
Article15. Zugel U, Sponaas AM, Neckermann J, Schoel B, Kaufmann SH. gp96-peptide vaccination of mice against intracellular bacteria. Infect Immun. 2001; 69:4164–4167.
Article16. Sugawara I, Udagawa T, Taniyama T. Protective efficacy of recombinant (Ag85A) BCG Tokyo with Ag85A peptide boosting against Mycobacterium tuberculosis-infected guinea pigs in comparison with that of DNA vaccine encoding Ag85A. Tuberculosis (Edinb). 2007; 87:94–101.
Article17. Gowthaman U, Rai PK, Khan N, Jackson DC, Agrewala JN. Lipidated promiscuous peptides vaccine for tuberculosis-endemic regions. Trends Mol Med. 2012; 18:607–614.
Article18. Bosio CM, Gardner D, Elkins KL. Infection of B cell-deficient mice with CDC 1551, a clinical isolate of Mycobacterium tuberculosis: delay in dissemination and development of lung pathology. J Immunol. 2000; 164:6417–6425.
Article19. Abebe F, Bjune G. The protective role of antibody responses during Mycobacterium tuberculosis infection. Clin Exp Immunol. 2009; 157:235–243.
Article20. Zuniga J, Torres-Garcia D, Santos-Mendoza T, Rodriguez-Reyna TS, Granados J, Yunis EJ. Cellular and humoral mechanisms involved in the control of tuberculosis. Clin Dev Immunol. 2012; 2012:193923.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of immunological markers and booster effects of Ag85B peptides, Ag85B, and BCG in blood of BCG vaccinated children: a preliminary report
- Construction of Recombinant BCGs Overexpressing Antigen 85 Complex and Their Protective Efficacy against Mycobacterium tuberculosis Infection in a Mouse Model
- Mycobacterium bovis Bacillus Calmette-Guerin (BCG) and BCG-based Vaccines Against Tuberculosis
- Development of a New Approach to Determine the Potency of Bacille Calmette–Guérin Vaccines Using Flow Cytometry
- Efficiency of Recombinant Bacille Calmette-Guerin in Inducing Humoral and Cell Mediated Immunities against Human Immunodeficiency Virus Type 1 Third Variable Domain in Immunized Mice