Clin Exp Vaccine Res.
2016 Jan;5(1):31-40. 10.7774/cevr.2016.5.1.31.
Assessment of immunological markers and booster effects of Ag85B peptides, Ag85B, and BCG in blood of BCG vaccinated children: a preliminary report
- Affiliations
-
- 1Biochemistry Research Laboratory, Central India Institute of Medical Sciences, Nagpur, India. raj_ciims@rediffmail.com
- KMID: 2152695
- DOI: http://doi.org/10.7774/cevr.2016.5.1.31
Abstract
- PURPOSE
In the present study, the protective immunological markers in serum and peripheral blood mononuclear cells (PBMCs) of bacillus Calmette-Guerin (BCG) vaccinated and unvaccinated children were evaluated after vaccination. Further, PBMCs of children with low protective levels were boosted with BCG, Ag85B, and Ag85B peptides to study their booster effects to increase waning BCG induced immunity.
MATERIALS AND METHODS
Fifty children from 1 month to 18 years of age were randomized for the study. Blood samples were collected from 27 participants with/without BCG vaccination. Immunological markers (anti-BCG, interferon gamma [IFN-gamma], and adenosine deaminase activity) were assessed in both serum and PBMCs of children. Children with low levels of protective immunological markers were further recruited and their PBMCs were boosted with BCG, Ag85B, and Ag85B peptides.
RESULTS
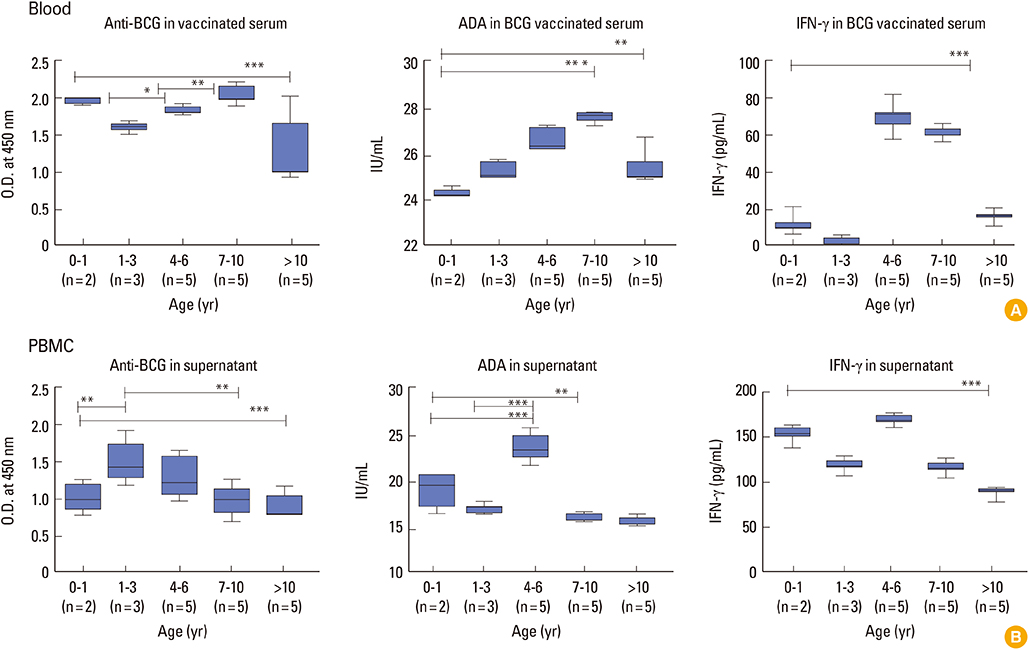
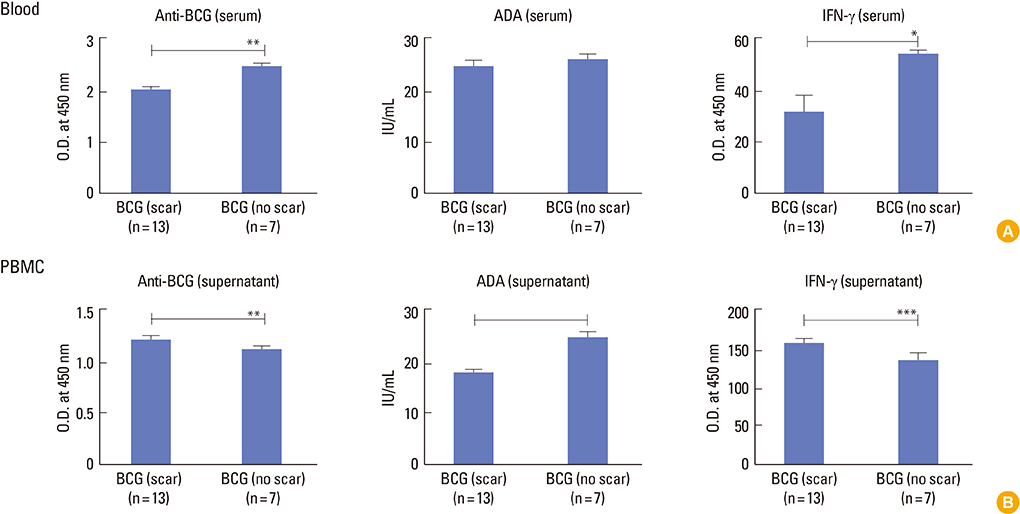
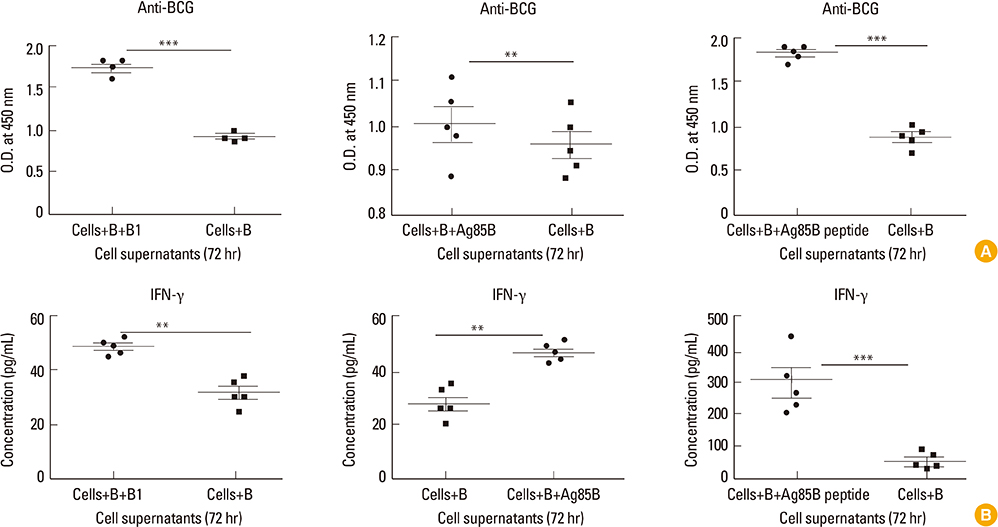
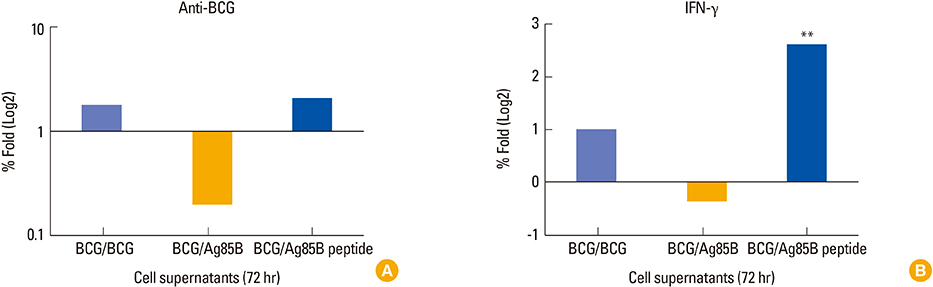
Children in age group of 4-6 years were associated with significantly (p<0.05) higher BCG-specific IgG and IFN-gamma levels compared to those in age group greater than 10 years. Vaccinated children had greater repertoire of immunological memory which on in vitro stimulation with BCG showed increase in BCG-specific response compared to unvaccinated controls. Assessment of booster effects of BCG, Ag85B, and Ag85B peptides in PBMCs of children revealed greater potential of peptides to boost BCG induced immunity compared to BCG and Ag85B.
CONCLUSION
To conclude, children within age 4-6 years are associated with high immunological markers which eventually diminish with age thereby suggesting need for booster dose in later years. Mycobacterium tuberculosis peptides along with BCG may be used as attractive candidates to boost such waning BCG induced immunity in children.
Keyword
MeSH Terms
Figure
Reference
-
1. Husain AA, Kashyap RS, Kalorey DR, et al. Effect of repeat dose of BCG vaccination on humoral response in mice model. Indian J Exp Biol. 2011; 49:7–10.2. Pereira SM, Dantas OM, Ximenes R, Barreto ML. BCG vaccine against tuberculosis: its protective effect and vaccination policies. Rev Saude Publica. 2007; 41:Suppl 1. 59–66.3. Dara M, Acosta CD, Rusovich V, et al. Bacille Calmette-Guerin vaccination: the current situation in Europe. Eur Respir J. 2014; 43:24–35.
Article4. Hanekom WA. The immune response to BCG vaccination of newborns. Ann N Y Acad Sci. 2005; 1062:69–78.
Article5. Raja A. Immunology of tuberculosis. Indian J Med Res. 2004; 120:213–232.6. Andersen P, Doherty TM. The success and failure of BCG: implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005; 3:656–662.
Article7. Husain AA, Warke SR, Kalorey DR, Daginawala HF, Taori GM, Kashyap RS. Comparative evaluation of booster efficacies of BCG, Ag85B, and Ag85B peptides based vaccines to boost BCG induced immunity in BALB/c mice: a pilot study. Clin Exp Vaccine Res. 2015; 4:83–87.
Article8. Kashyap RS, Husain AA, Morey SH, et al. Assessment of immune response to repeat stimulation with BCG vaccine using in vitro PBMC model. J Immune Based Ther Vaccines. 2010; 8:3.
Article9. Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011; 8:e1001012.
Article10. Colditz GA, Berkey CS, Mosteller F, et al. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995; 96(1 Pt 1):29–35.
Article11. Tidjani O, Amedome A, ten Dam HG. The protective effect of BCG vaccination of the newborn against childhood tuberculosis in an African community. Tubercle. 1986; 67:269–281.
Article12. Young TK, Hershfield ES. A case-control study to evaluate the effectiveness of mass neonatal BCG vaccination among Canadian Indians. Am J Public Health. 1986; 76:783–786.
Article13. Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011; 10:645–658.
Article14. Briassoulis G, Karabatsou I, Gogoglou V, Tsorva A. BCG vaccination at three different age groups: response and effectiveness. J Immune Based Ther Vaccines. 2005; 3:1.15. Gowthaman U, Mushtaq K, Tan AC, Rai PK, Jackson DC, Agrewala JN. Challenges and solutions for a rational vaccine design for TB-endemic regions. Crit Rev Microbiol. 2015; 41:389–398.
Article16. Gowthaman U, Rai PK, Khan N, Jackson DC, Agrewala JN. Lipidated promiscuous peptides vaccine for tuberculosis-endemic regions. Trends Mol Med. 2012; 18:607–614.
Article17. Brandt L, Feino Cunha J, Weinreich Olsen A, et al. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun. 2002; 70:672–678.
Article18. Barreto ML, Pereira SM, Ferreira AA. BCG vaccine: efficacy and indications for vaccination and revaccination. J Pediatr (Rio J). 2006; 82:3 Suppl. S45–S54.
Article19. Rodrigues LC, Pereira SM, Cunha SS, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005; 366:1290–1295.
Article20. Whelan KT, Pathan AA, Sander CR, et al. Safety and immunogenicity of boosting BCG vaccinated subjects with BCG: comparison with boosting with a new TB vaccine, MVA85A. PLoS One. 2009; 4:e5934.
Article21. Hoft DF. Development of vaccines to control the worldwide tuberculosis pandemic. Mo Med. 2014; 111:326–331.22. Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009; 21:346–351.
Article23. Narayanan PR. Influence of sex, age and nontuberculous infection at intake on the efficacy of BCG: re-analysis of 15-year data from a double-blind randomized control trial in South India. Indian J Med Res. 2006; 123:119–124.24. Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. 2007; 6:404–414.
Article25. Chodisetti SB, Rai PK, Gowthaman U, Pahari S, Agrewala JN. Potential T cell epitopes of Mycobacterium tuberculosis that can instigate molecular mimicry against host: implications in autoimmune pathogenesis. BMC Immunol. 2012; 13:13.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative evaluation of booster efficacies of BCG, Ag85B, and Ag85B peptides based vaccines to boost BCG induced immunity in BALB/c mice: a pilot study
- Construction of Recombinant BCGs Overexpressing Antigen 85 Complex and Their Protective Efficacy against Mycobacterium tuberculosis Infection in a Mouse Model
- The Current Status of BCG Vaccination in Young Children in South Korea
- BCG Osteomyelitis: A Case Report
- Tuberculin Reactivity in Neonates Vaccinated with BCG at Primary Care Clinics: With Two Types of BCG Vaccine and Two Strengths of PPD