J Korean Neurosurg Soc.
2014 Nov;56(5):383-389. 10.3340/jkns.2014.56.5.383.
Enhanced Efficacy of Human Brain-Derived Neural Stem Cells by Transplantation of Cell Aggregates in a Rat Model of Parkinson's Disease
- Affiliations
-
- 1Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. srjeon@amc.seoul.kr
- 2Department of Biochemistry and Molecular Biology, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Maxillofacial Biomedical Engineering, Institute of Oral Biology, School of Dentistry, Kyung Hee University, Seoul, Korea.
- 4Center for Bionics of Korea Institute of Science and Technology, Seoul, Korea.
- 5Department of Neurosurgery, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 2018095
- DOI: http://doi.org/10.3340/jkns.2014.56.5.383
Abstract
OBJECTIVE
Neural tissue transplantation has been a promising strategy for the treatment of Parkinson's disease (PD). However, transplantation has the disadvantages of low-cell survival and/or development of dyskinesia. Transplantation of cell aggregates has the potential to overcome these problems, because the cells can extend their axons into the host brain and establish synaptic connections with host neurons. In this present study, aggregates of human brain-derived neural stem cells (HB-NSC) were transplanted into a PD animal model and compared to previous report on transplantation of single-cell suspensions.
METHODS
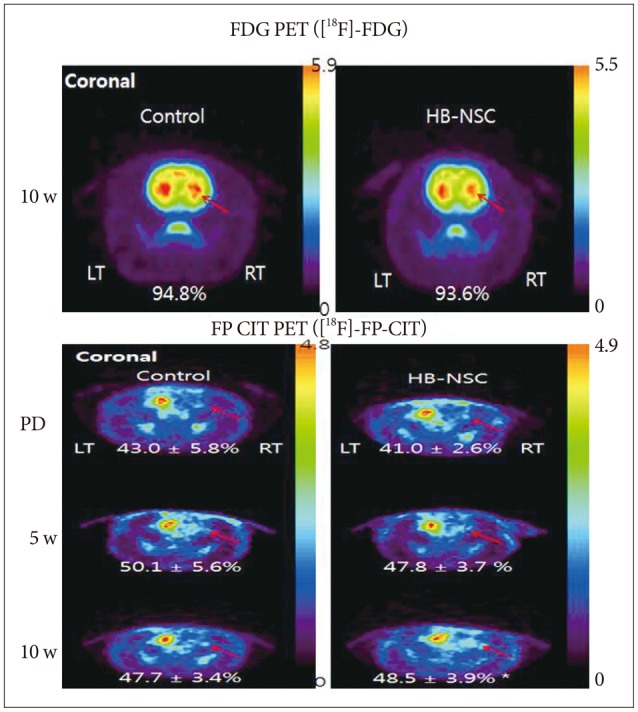
Rats received an injection of 6-OHDA into the right medial forebrain bundle to generate the PD model and followed by injections of PBS only, or HB-NSC aggregates in PBS into the ipsilateral striatum. Behavioral tests, multitracer (2-deoxy-2-[18F]-fluoro-D-glucose ([18F]-FDG) and [18F]-N-(3-fluoropropyl)-2-carbomethoxy-3-(4-iodophenyl)nortropane ([18F]-FP-CIT) microPET scans, as well as immunohistochemical (IHC) and immunofluorescent (IF) staining were conducted to evaluate the results.
RESULTS
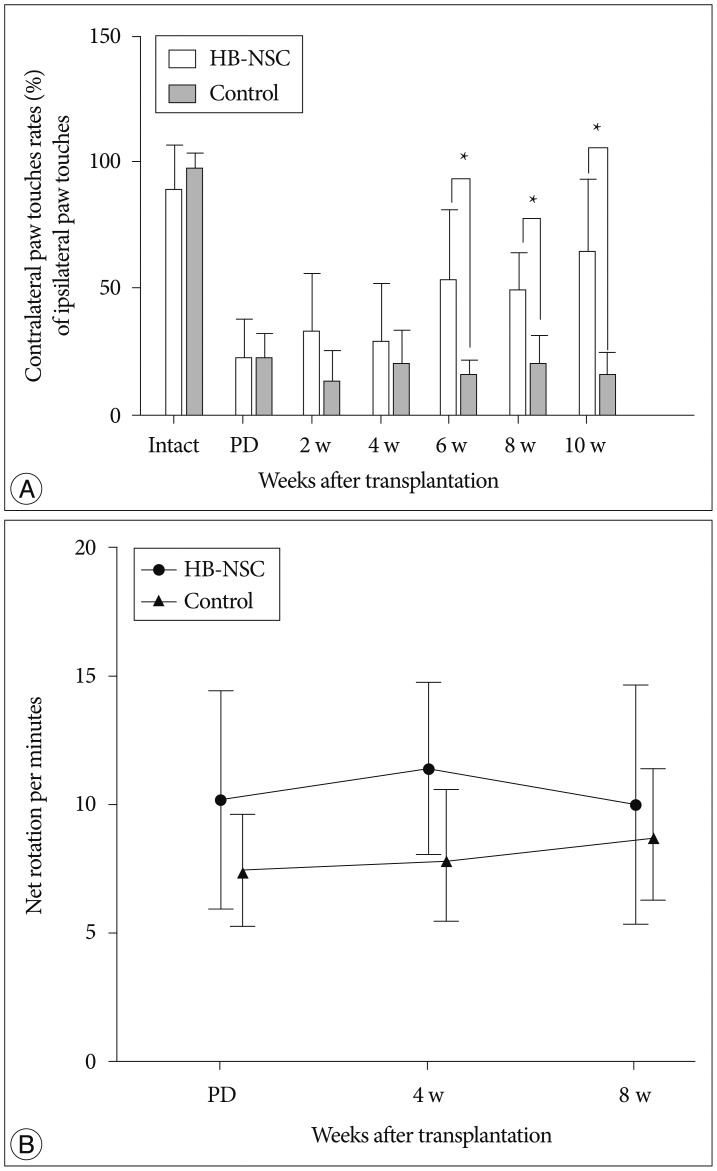
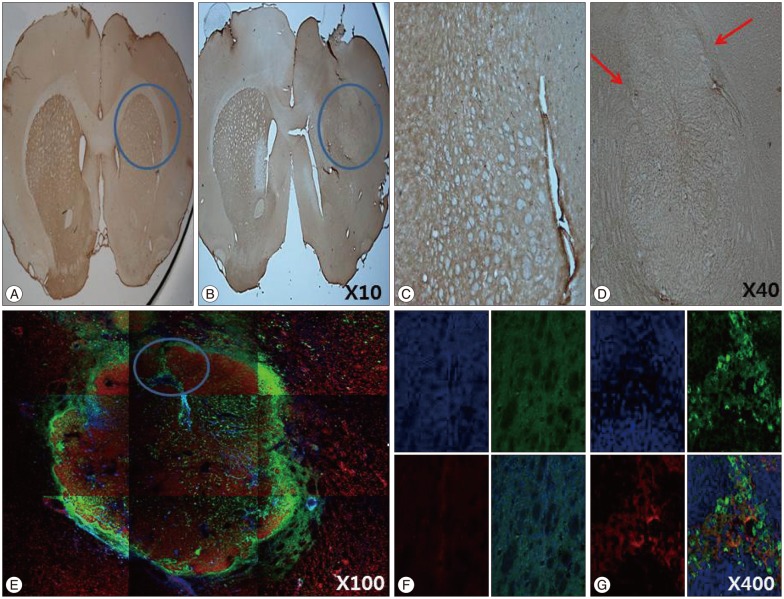
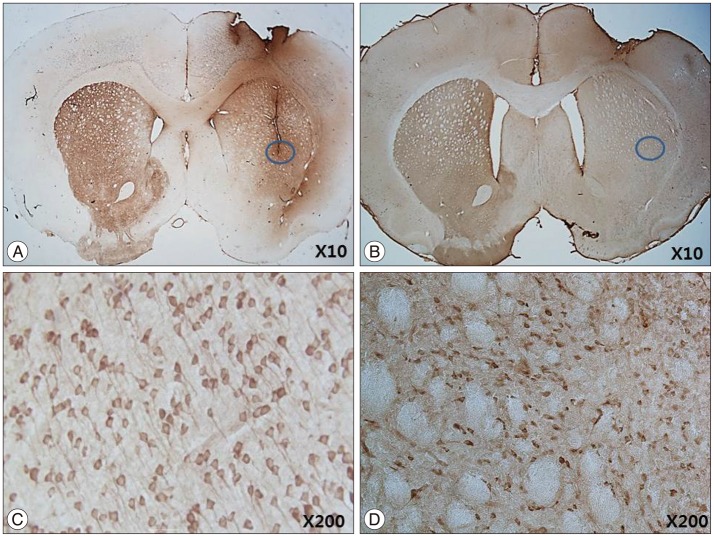
The stepping test showed significant improvement of contralateral forelimb control in the HB-NSC group from 6-10 weeks compared to the control group (p<0.05). [18F]-FP-CIT microPET at 10 weeks posttransplantation demonstrated a significant increase in uptake in the HB-NSC group compared to pretransplantation (p<0.05). In IHC and IF staining, tyrosine hydroxylase and human beta2 microglobulin (a human cell marker) positive cells were visualized at the transplant site.
CONCLUSION
These results suggest that the HB-NSC aggregates can survive in the striatum and exert therapeutic effects in a PD model by secreting dopamine.
Keyword
MeSH Terms
Figure
Reference
-
1. Agid Y. Parkinson's disease : pathophysiology. Lancet. 1991; 337:1321–1324. PMID: 1674304.2. Björklund A, Dunnett SB, Stenevi U, Lewis ME, Iversen SD. Reinnervation of the denervated striatum by substantia nigra transplants : functional consequences as revealed by pharmacological and sensorimotor testing. Brain Res. 1980; 199:307–333. PMID: 7417786.
Article3. Capirci C, Rampin L, Erba PA, Galeotti F, Crepaldi G, Banti E, et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging. 2007; 34:1583–1593. PMID: 17503039.
Article4. Dauer W, Przedborski S. Parkinson's disease : mechanisms and models. Neuron. 2003; 39:889–909. PMID: 12971891.5. Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003; 302:819–822. PMID: 14593166.
Article6. Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1992; 55:138–142. PMID: 1538221.
Article7. Downes JH, Hammond MW, Xydas D, Spencer MC, Becerra VM, Warwick K, et al. Emergence of a small-world functional network in cultured neurons. PLoS Comput Biol. 2012; 8:e1002522. PMID: 22615555.
Article8. Erdö F, Bührle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003; 23:780–785. PMID: 12843782.
Article9. Hagell P, Piccini P, Björklund A, Brundin P, Rehncrona S, Widner H, et al. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci. 2002; 5:627–628. PMID: 12042822.
Article10. Hedlund E, Perlmann T. Neuronal cell replacement in Parkinson's disease. J Intern Med. 2009; 266:358–371. PMID: 19765180.
Article11. Hwang O, Baker H, Gross S, Joh TH. Localization of GTP cyclohydrolase in monoaminergic but not nitric oxide-producing cells. Synapse. 1998; 28:140–153. PMID: 9450514.
Article12. Bjorklund A, Dunnett SB, Brundin P, Stoessl AJ, Freed CR, Breeze RE, et al. Neural transplantation for the treatment of Parkinson's disease. Lancet Neurol. 2003; 2:437–445. PMID: 12849125.
Article13. Kato-Negishi M, Tsuda Y, Onoe H, Takeuchi S. A neurospheroid network-stamping method for neural transplantation to the brain. Biomaterials. 2010; 31:8939–8945. PMID: 20850180.
Article14. Kazumata K, Dhawan V, Chaly T, Antonini A, Margouleff C, Belakhlef A, et al. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med. 1998; 39:1521–1530. PMID: 9744335.15. Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004; 101:11839–11844. PMID: 15280535.
Article16. Kim JS, Lee JS, Im KC, Kim SJ, Kim SY, Lee DS, et al. Performance measurement of the microPET focus 120 scanner. J Nucl Med. 2007; 48:1527–1535. PMID: 17704248.
Article17. Kim ST, Choi JH, Chang JW, Kim SW, Hwang O. Immobilization stress causes increases in tetrahydrobiopterin, dopamine, and neuromelanin and oxidative damage in the nigrostriatal system. J Neurochem. 2005; 95:89–98. PMID: 16181415.
Article18. Lei Z, Jiang Y, Li T, Zhu J, Zeng S. Signaling of glial cell line-derived neurotrophic factor and its receptor GFRα1 induce Nurr1 and Pitx3 to promote survival of grafted midbrain-derived neural stem cells in a rat model of Parkinson disease. J Neuropathol Exp Neurol. 2011; 70:736–747. PMID: 21865882.
Article19. Lund RD, Hauschka SD. Transplanted neural tissue develops connections with host rat brain. Science. 1976; 193:582–584. PMID: 959815.
Article20. McNaught KS, Belizaire R, Jenner P, Olanow CW, Isacson O. Selective loss of 20S proteasome alpha-subunits in the substantia nigra pars compacta in Parkinson's disease. Neurosci Lett. 2002; 326:155–158. PMID: 12095645.
Article21. Mehta V, Spears J, Mendez I. Neural transplantation in Parkinson's disease. Can J Neurol Sci. 1997; 24:292–301. PMID: 9398975.
Article22. Nikkhah G, Cunningham MG, Cenci MA, McKay RD, Björklund A. Dopaminergic microtransplants into the substantia nigra of neonatal rats with bilateral 6-OHDA lesions. I. Evidence for anatomical reconstruction of the nigrostriatal pathway. J Neurosci. 1995; 15(5 Pt 1):3548–3561. PMID: 7538563.
Article23. Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson's disease. Trends Neurosci. 1996; 19:102–109. PMID: 9054056.
Article24. Paillé V, Henry V, Lescaudron L, Brachet P, Damier P. Rat model of Parkinson's disease with bilateral motor abnormalities, reversible with levodopa, and dyskinesias. Mov Disord. 2007; 22:533–539. PMID: 17230470.
Article25. Puschban Z, Scherfler C, Granata R, Laboyrie P, Quinn NP, Jenner P, et al. Autoradiographic study of striatal dopamine re-uptake sites and dopamine D1 and D2 receptors in a 6-hydroxydopamine and quinolinic acid double-lesion rat model of striatonigral degeneration (multiple system atrophy) and effects of embryonic ventral mesencephalic, striatal or co-grafts. Neuroscience. 2000; 95:377–388. PMID: 10658617.
Article26. Rehncrona S. A critical review of the current status and possible developments in brain transplantation. Adv Tech Stand Neurosurg. 1997; 23:3–46. PMID: 9075470.
Article27. Tabbal S, Fahn S, Frucht S. Fetal tissue transplantation [correction of transplanation] in Parkinson's disease. Curr Opin Neurol. 1998; 11:341–349. PMID: 9725080.28. Williams LN, Seignourel P, Crucian GP, Okun MS, Rodriguez RL, Skidmore FM, et al. Laterality, region, and type of motor dysfunction correlate with cognitive impairment in Parkinson's disease. Mov Disord. 2007; 22:141–145. PMID: 17089386.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prospect of cell therapy for Parkinson's disease
- Development and application of neural stem cells for treating various human neurological diseases in animal models
- Direct Differentiation of Adult Ocular Progenitors into Striatal Dopaminergic Neurons
- Expression of mRNAs for Neurotrophic Factors in Human Neural Stem Cells Derived from Fetal Telencephalon
- Differentiation of Rat Neural Stem Cells Following Transplantation in the Brain of Huntington's Disease Rat Model