J Korean Diabetes Assoc.
2007 Mar;31(2):105-112. 10.4093/jkda.2007.31.2.105.
Mechanism of 2-Deoxy-D-ribose-induced Damage in Pancreatic beta-cells
- Affiliations
-
- 1Department of Internal Medicine, Cheju National University College of Medicine, Korea.
- 2Department of Medicine1, Cheju Nationa University College of Medicine, Korea.
- 3Department of Endocrinology & Metabolism, Kyung Hee University College of Medicine, Korea.
- KMID: 2008078
- DOI: http://doi.org/10.4093/jkda.2007.31.2.105
Abstract
-
BACKGROUND: Mechanism for glucose toxicity is known to be an increased oxidative stress produced by multiple pathways. In our previous report, 2-deoxy-d-ribose (dRib) promoted apoptosis by increasing oxidative stress in a pancreatic beta-cell line. We performed this study to investigate the mechanism of dRib-induced damage of beta-cells.
METHODS
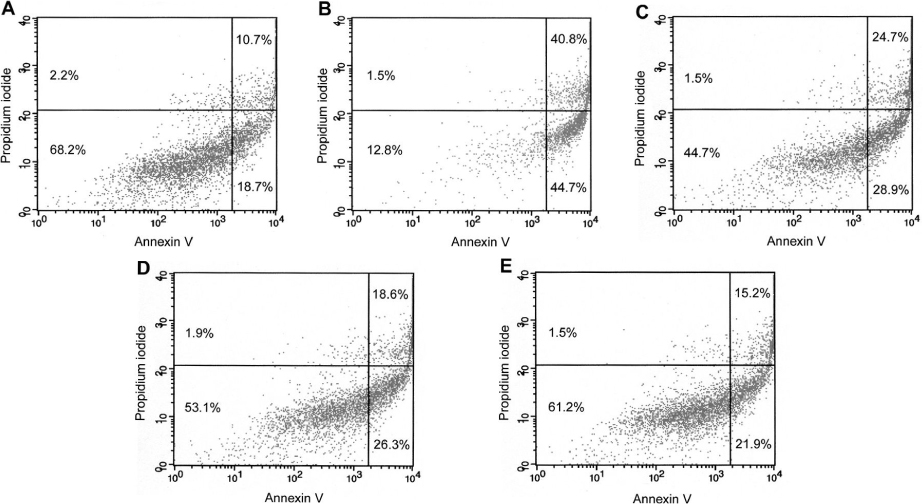
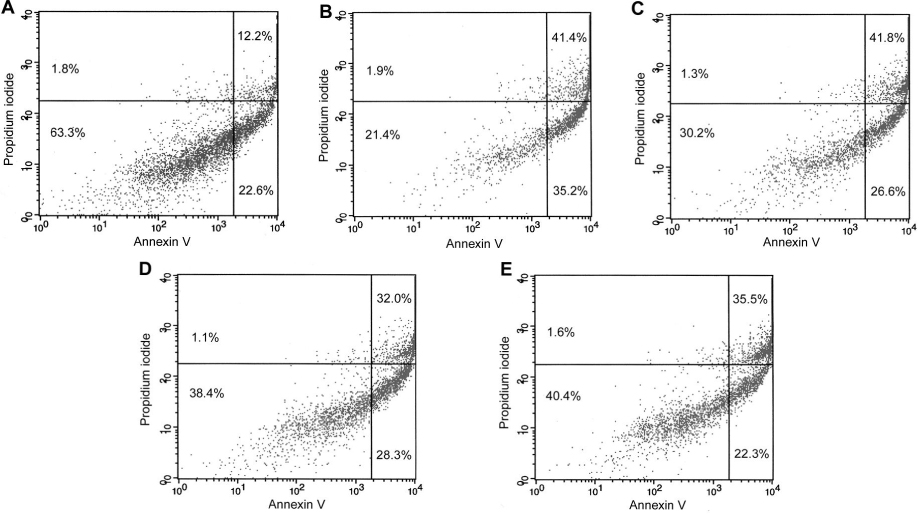
HIT-T15 cells were cultured in RPMI-1640 medium with 40 mM dRib for 24 hours after pretreatment with various concentrations of a metal chelator (DTPA) and inhibitors of protein glycation (aminoguanidine and pyridoxamine). Cell viability was determined by MTT assay. Apoptosis was analyzed by flow cytometry with annexin V/PI double staining.
RESULTS
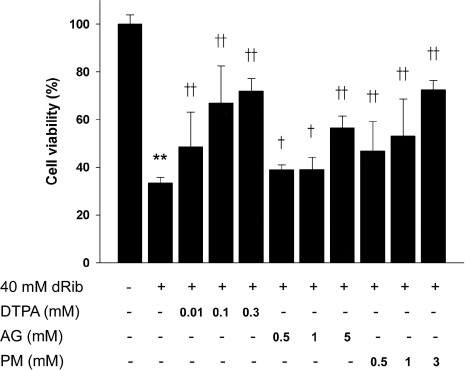
DTPA, which inhibits the monosaccharide autoxidation, partially reversed dRib-induced cytotoxicity in a dose-dependent manner (P < 0.01). The cytotoxicity was also suppressed dose-dependently by aminoguanidine (AG) and pyridoxamine (PM) (P < 0.05 and P < 0.01, repectively). Flow cytometric analysis showed that pretreatment of DTPA and AG also reversed the dRib-triggered apoptosis in a dose-dependent manner. We assessed the additional protective effects of inhibitors of protein glycation from dRib-induced cytotoxiciy in the presence of a metal chelator. The additions of AG (P < 0.05) and PM (P < 0.01) significantly reduced the cytotoxicity compared with DTPA alone group.
CONCLUSION
This results suggest that dRib produce cytotoxicity and apoptosis through the mechanisms of advanced glycation endproducts (AGEs) formation including the monsaccharide autoxidation and protein glycation in pancreatic beta-cell. Thus, dRib could be a surrogate for glucose in the study of glucose toxicity and chronic diabetic complications.
Keyword
MeSH Terms
Figure
Reference
-
1. Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001. 50:Suppl 1. S154–S159.2. Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990. 13:610–630.3. McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999. 42:128–138.4. Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994. 368:756–760.5. Polonsky KS, Sturis J, Bell GI. Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulindependent diabetes mellitus-a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med. 1996. 334:777–783.6. Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003. 52:581–587.7. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001. 414:813–820.8. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004. 279:42351–42354.9. Hodge JE. Dehydrated foods: Chemistry of browning reactions in model systems. Agric Food Chem. 1953. 1:928–943.10. Monnier VM. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J Gerontol. 1990. 45:B105–B111.11. Khalifah RG, Baynes JW, Hudson BG. Amadorins: novel post-Amadori inhibitors of advanced glycation reactions. Biochem Biophys Res Commun. 1999. 257:251–258.12. Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988. 256:205–212.13. Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of 'autoxidative' glycosylation in diabetes. Biochem J. 1987. 245:243–250.14. Beisswenger PJ, Howell SK, Nelson RG, Mauer M, Szwergold BS. Alpha-oxoaldehyde metabolism and diabetic complications. Biochem Soc Tran. 2003. 31:1358–1363.15. Bunn HF, Higgins PJ. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981. 213:222–224.16. Olson LK, Redmon JB, Towle HC, Robertson RP. Chronic exposure of HIT cells to high glucose concentrations paradoxically decreases insulin gene transcription and alters binding of insulin gene regulatory protein. J Clin Invest. 1993. 92:514–519.17. Koh G, Suh KS, Chon S, Oh S, Woo JT, Kim SW, Kim JW, Kim YS. Elevated cAMP level attenuates 2-deoxy-d-ribose-induced oxidative damage in pancreatic beta-cells. Arch Biochem Biophys. 2005. 438:70–79.18. Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987. 47:936–942.19. Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994. 84:1415–1420.20. Price DL, Rhett PM, Thorpe SR, Baynes JW. Chelating activity of advanced glycation end-product inhibitors. J Biol Chem. 2001. 276:48967–48972.21. Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995. 34:3702–3709.22. Thornalley P, Wolff S, Crabbe J, Stern A. The autoxidation of glyceraldehyde and other simple monosaccharides under physiological conditions catalysed by buffer ions. Biochim Biophys Acta. 1984. 797:276–287.23. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991. 40:405–412.24. Hunt JV, Bottoms MA, Mitchinson MJ. Oxidative alterations in the experimental glycation model of diabetes mellitus are due to protein-glucose adduct oxidation. Some fundamental differences in proposed mechanisms of glucose oxidation and oxidant production. Biochem J. 1993. 291(Pt 2):529–535.25. Fu MX, Wells-Knecht KJ, Blackledge JA, Lyons TJ, Thorpe SR, Baynes JW. Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes. 1994. 43:676–683.26. Wells-Knecht MC, Thorpe SR, Baynes JW. Pathways of formation of glycoxidation products during glycation of collagen. Biochemistry. 1995. 34:15134–15141.27. Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003. 419:31–40.28. Nilsson BO. Biological effects of aminoguanidine: an update. Inflamm Res. 1999. 48:509–515.29. Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci U S A. 1999. 96:10857–10862.30. Voziyan PA, Hudson BG. Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell Mol Life Sci. 2005. 62:1671–1681.31. Khalifah RG, Todd P, Booth AA, Yang SX, Mott JD, Hudson BG. Kinetics of nonenzymatic glycation of ribonuclease A leading to advanced glycation end products. Paradoxical inhibition by ribose leads to facile isolation of protein intermediate for rapid post-Amadori studies. Biochemistry. 1996. 35:4645–4654.32. Booth AA, Khalifah RG, Todd P, Hudson BG. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs). Novel inhibition of post-Amadori glycation pathways. J Biol Chem. 1997. 272:5430–5437.33. Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, Thorpe SR, Baynes JW. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002. 61:939–950.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oxidative Stress of INS-1 Cell, HIT-T15 Cell and Rat Islet Cell as a Mechanism of Glucose Toxicity
- Protective Effects of Glucagon Like Peptide-1 on HIT-T15 beta Cell Apoptosis via ER Stress Induced by 2-deoxy-D-glucose
- Protective mechanism of glucose against alloxan-induced beta-cell damage: pivotal role of ATP
- Effect of Intracellular ATP on Zn2+ Blockade of KATP Channels in Pancreatic Beta Cells
- Oxidative Stress and Cell Dysfunction in Diabetes: Role of ROS Produced by Mitochondria and NAD(P)H Oxidase