J Korean Assoc Oral Maxillofac Surg.
2014 Aug;40(4):173-180. 10.5125/jkaoms.2014.40.4.173.
Neurogenic differentiation of human dental stem cells in vitro
- Affiliations
-
- 1Biotooth Engineering Lab, Department of Oral and Maxillofacial Surgery and Craniomaxillofacial Life Science, Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea. seobm@snu.ac.kr
- 2Dental Regenerative Biotechnology, Department of Dental Science, School of Dentistry, Seoul National University, Seoul, Korea.
- 3Division of Oral and Maxillofacial Surgery, Department of Dentistry, Korea University Anam Hospital, Seoul, Korea.
- KMID: 2005442
- DOI: http://doi.org/10.5125/jkaoms.2014.40.4.173
Abstract
OBJECTIVES
The purpose of this study was to investigate the neurogenic differentiation of human dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs), and stem cells from apical papilla (SCAP).
MATERIALS AND METHODS
After induction of neurogenic differentiation using commercial differentiation medium, expression levels of neural markers, microtubule-associated protein 2 (MAP2), class III beta-tubulin, and glial fibrillary acidic protein (GFAP) were identified using reverse transcriptase polymerase chain reaction (PCR), real-time PCR, and immunocytochemistry.
RESULTS
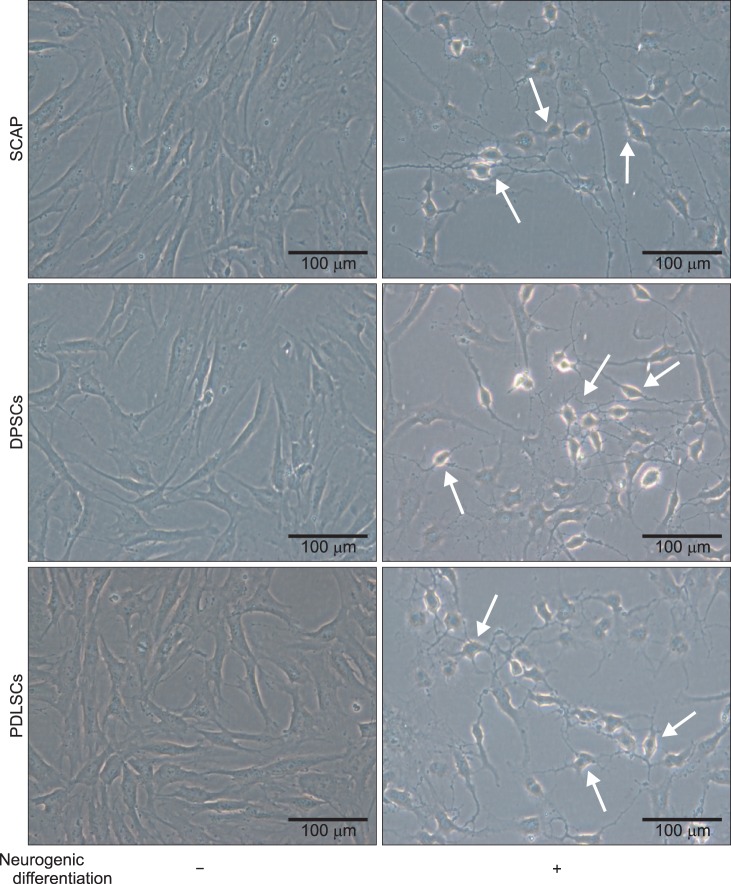
The induced cells showed neuron-like morphologies, similar to axons, dendrites, and perikaryons, which are composed of neurons in DPSCs, PDLSCs, and SCAP. The mRNA levels of neuronal markers tended to increase in differentiated cells. The expression of MAP2 and beta-tubulin III also increased at the protein level in differentiation groups, even though GFAP was not detected via immunocytochemistry.
CONCLUSION
Human dental stem cells including DPSCs, PDLSCs, and SCAP may have neurogenic differentiation capability in vitro. The presented data support the use of human dental stem cells as a possible alternative source of stem cells for therapeutic utility in the treatment of neurological diseases.
MeSH Terms
-
Axons
Dendrites
Dental Papilla
Dental Pulp
Glial Fibrillary Acidic Protein
Humans
Immunohistochemistry
Microtubule-Associated Proteins
Neurons
Periodontal Ligament
Real-Time Polymerase Chain Reaction
Reverse Transcriptase Polymerase Chain Reaction
RNA, Messenger
Stem Cells*
Tubulin
Glial Fibrillary Acidic Protein
Microtubule-Associated Proteins
RNA, Messenger
Tubulin
Figure
Reference
-
1. Duan X, Tu Q, Zhang J, Ye J, Sommer C, Mostoslavsky G, et al. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol. 2011; 226:150–157. PMID: 20658533.
Article2. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007; 100:1249–1260. PMID: 17495232.
Article3. Jones E, Yang X. Mesenchymal stem cells and bone regeneration: current status. Injury. 2011; 42:562–568. PMID: 21489533.
Article4. Malliaras K, Marbán E. Cardiac cell therapy: where we've been, where we are, and where we should be headed. Br Med Bull. 2011; 98:161–185. PMID: 21652595.
Article5. Ohba S, Ikeda T, Kugimiya F, Yano F, Lichtler AC, Nakamura K, et al. Identification of a potent combination of osteogenic genes for bone regeneration using embryonic stem (ES) cell-based sensor. FASEB J. 2007; 21:1777–1787. PMID: 17317722.
Article6. Dangaria SJ, Ito Y, Walker C, Druzinsky R, Luan X, Diekwisch TG. Extracellular matrix-mediated differentiation of periodontal progenitor cells. Differentiation. 2009; 78:79–90. PMID: 19433344.
Article7. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000; 97:13625–13630. PMID: 11087820.8. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003; 100:5807–5812. PMID: 12716973.
Article9. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155. PMID: 15246727.
Article10. Lee JH, Um S, Jang JH, Seo BM. Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res. 2012; 348:475–484. PMID: 22437875.
Article11. Um S, Choi JR, Lee JH, Zhang Q, Seo B. Effect of leptin on differentiation of human dental stem cells. Oral Dis. 2011; 17:662–669. PMID: 21702867.
Article12. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006; 1:e79. PMID: 17183711.
Article13. Komada Y, Yamane T, Kadota D, Isono K, Takakura N, Hayashi S, et al. Origins and properties of dental, thymic, and bone marrow mesenchymal cells and their stem cells. PLoS One. 2012; 7:e46436. PMID: 23185234.
Article14. Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000; 127:1671–1679. PMID: 10725243.
Article15. Abe S, Hamada K, Miura M, Yamaguchi S. Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol Int. 2012; 36:927–936. PMID: 22731688.
Article16. Karaöz E, Demircan PC, Sağlam O, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol. 2011; 136:455–473. PMID: 21879347.
Article17. Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009; 87:2183–2200. PMID: 19301431.
Article18. Völlner F, Ernst W, Driemel O, Morsczeck C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation. 2009; 77:433–441. PMID: 19394129.
Article19. Nourbakhsh N, Soleimani M, Taghipour Z, Karbalaie K, Mousavi SB, Talebi A, et al. Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells. Int J Dev Biol. 2011; 55:189–195. PMID: 21671222.
Article20. Aanismaa R, Hautala J, Vuorinen A, Miettinen S, Narkilahti S. Human dental pulp stem cells differentiate into neural precursors but not into mature functional neurons. Stem Cell Discov. 2012; 2:85–91.
Article21. Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012; 122:80–90. PMID: 22133879.
Article22. de Almeida FM, Marques SA, Ramalho Bdos S, Rodrigues RF, Cadilhe DV, Furtado D, et al. Human dental pulp cells: a new source of cell therapy in a mouse model of compressive spinal cord injury. J Neurotrauma. 2011; 28:1939–1949. PMID: 21609310.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- Modulation of osteoblastic/odontoblastic differentiation of adult mesenchymal stem cells through gene introduction: a brief review
- The Osteogenic Role of Biomaterials Combined with HumanDerived Dental Stem Cells in Bone Tissue Regeneration
- Transcriptional Profiles of Imprinted Genes in Human Embryonic Stem Cells During In vitro Differentiation
- Effects of nanoscale ridge/groovepattern arrayed surface on in vitro differentiation of multi-potent pulp cells derived from human supernumerary teeth