J Korean Soc Transplant.
2012 Sep;26(3):165-173. 10.4285/jkstn.2012.26.3.165.
The Role of Macrophages in Transplant Rejection
- Affiliations
-
- 1Transplantation Research Institute, Seoul National University College of Medicine, Seoul, Korea. jcyjs@dreamwiz.com
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Transplantation Center, Seoul National University Hospital, Seoul, Korea.
- KMID: 2003531
- DOI: http://doi.org/10.4285/jkstn.2012.26.3.165
Abstract
- Macrophage accumulation has been recognized as a feature of allograft rejection, however, the role of macrophages in rejection remains underappreciated. Macrophages are present within graft tissues throughout the lifespan of the graft, including acute rejection episodes. Recent advances in macrophage biology have demonstrated that different types of macrophages in grafts serve a range of functions, including promotion or attenuation of inflammation, participation in innate and adaptive immune responses, and mediation of tissue injury, fibrosis, and tissue repair. Macrophages contribute to both the innate and acquired arms of the alloimmune response, and, thus, may be involved in all aspects of acute and chronic allograft rejection. Macrophages are also involved in hyperacute and acute vascular rejection of xenografts. A deeper understanding of how macrophages accumulate within grafts and of the factors that control differentiation and function of these cells could lead to identification of novel therapeutic targets in transplantation.
Keyword
MeSH Terms
Figure
Reference
-
1. Brent L, Brown J, Medawar PB. Skin transplantation immunity in relation to hypersensitivity. Lancet. 1958. 2:561–564.
Article2. van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989. 79:125–150.
Article3. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000. 18:767–811.
Article4. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003. 3:23–35.
Article5. Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993. 259:1739–1742.
Article6. Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002. 277:15107–15112.
Article7. Fernández N, Renedo M, García-Rodríguez C, Sánchez Crespo M. Activation of monocytic cells through Fc gamma receptors induces the expression of macro-phage-inflammatory protein (MIP)-1 alpha, MIP-1 beta, and RANTES. J Immunol. 2002. 169:3321–3328.
Article8. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992. 176:287–292.
Article9. Doyle AG, Herbein G, Montaner LJ, Minty AJ, Caput D, Ferrara P, et al. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-gamma. Eur J Immunol. 1994. 24:1441–1445.
Article10. Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL. Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. J Biol Chem. 2002. 277:5082–5089.
Article11. Yada A, Ebihara S, Matsumura K, Endo S, Maeda T, Nakamura A, et al. Accelerated antigen presentation and elicitation of humoral response in vivo by FcgammaRIIB- and FcgammaRI/III-mediated immune complex uptake. Cell Immunol. 2003. 225:21–32.
Article12. Barker RN, Erwig LP, Hill KS, Devine A, Pearce WP, Rees AJ. Antigen presentation by macrophages is enhanced by the uptake of necrotic, but not apoptotic, cells. Clin Exp Immunol. 2002. 127:220–225.
Article13. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998. 101:890–898.
Article14. Matzinger P. The danger model: a renewed sense of self. Science. 2002. 296:301–305.
Article15. Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004. 173:7115–7119.
Article16. He H, Stone JR, Perkins DL. Analysis of robust innate immune response after transplantation in the absence of adaptive immunity. Transplantation. 2002. 73:853–861.
Article17. Grau V, Herbst B, Steiniger B. Dynamics of monocytes/macrophages and T lymphocytes in acutely rejecting rat renal allografts. Cell Tissue Res. 1998. 291:117–126.
Article18. Paradis IL, Marrari M, Zeevi A, Duquesnoy RJ, Griffith BP, Hardesty RL, et al. HLA phenotype of lung lavage cells following heart-lung transplantation. J Heart Transplant. 1985. 4:422–425.19. Penfield JG, Wang Y, Li S, Kielar MA, Sicher SC, Jeyarajah DR, et al. Transplant surgery injury recruits recipient MHC class II-positive leukocytes into the kidney. Kidney Int. 1999. 56:1759–1769.
Article20. Grimm PC, McKenna R, Nickerson P, Russell ME, Gough J, Gospodarek E, et al. Clinical rejection is distinguished from subclinical rejection by increased infiltration by a population of activated macrophages. J Am Soc Nephrol. 1999. 10:1582–1589.
Article21. Hancock WW, Thomson NM, Atkins RC. Composition of interstitial cellular infiltrate identified by monoclonal antibodies in renal biopsies of rejecting human renal allografts. Transplantation. 1983. 35:458–463.
Article22. Pilmore HL, Painter DM, Bishop GA, McCaughan GW, Eris JM. Early up-regulation of macrophages and myofibroblasts: a new marker for development of chronic renal allograft rejection. Transplantation. 2000. 69:2658–2662.
Article23. Grandaliano G, Gesualdo L, Ranieri E, Monno R, Stallone G, Schena FP. Monocyte chemotactic peptide-1 expression and monocyte infiltration in acute renal transplant rejection. Transplantation. 1997. 63:414–420.
Article24. Pattison J, Nelson PJ, Huie P, von Leuttichau I, Farshid G, Sibley RK, et al. RANTES chemokine expression in cell-mediated transplant rejection of the kidney. Lancet. 1994. 343:209–211.
Article25. Grau V, Gemsa D, Steiniger B, Garn H. Chemokine expression during acute rejection of rat kidneys. Scand J Immunol. 2000. 51:435–440.
Article26. Lan HY, Yang N, Brown FG, Isbel NM, Nikolic-Paterson DJ, Mu W, et al. Macrophage migration inhibitory factor expression in human renal allograft rejection. Transplantation. 1998. 66:1465–1471.
Article27. Scriba A, Grau V, Steiniger B. Phenotype of rat monocytes during acute kidney allograft rejection: increased expression of NKR-P1 and reduction of CD43. Scand J Immunol. 1998. 47:332–342.
Article28. Erwig LP, Rees AJ. Macrophage activation and programming and its role for macrophage function in glomerular inflammation. Kidney Blood Press Res. 1999. 22:21–25.
Article29. Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002. 3:143–150.
Article30. Kerr PG, Nikolic-Paterson DJ, Lan HY, Tesch G, Rainone S, Atkins RC. Deoxyspergualin suppresses local macrophage proliferation in rat renal allograft rejection. Transplantation. 1994. 58:596–601.
Article31. Lee I, Wang L, Wells AD, Ye Q, Han R, Dorf ME, et al. Blocking the monocyte chemoattractant protein-1/CCR2 chemokine pathway induces permanent survival of islet allografts through a programmed death-1 ligand-1-dependent mechanism. J Immunol. 2003. 171:6929–6935.
Article32. Le Meur Y, Jose MD, Mu W, Atkins RC, Chadban SJ. Macrophage colony-stimulating factor expression and macrophage accumulation in renal allograft rejection. Transplantation. 2002. 73:1318–1324.
Article33. Jose MD, Le Meur Y, Atkins RC, Chadban SJ. Blockade of macrophage colony-stimulating factor reduces macrophage proliferation and accumulation in renal allograft rejection. Am J Transplant. 2003. 3:294–300.
Article34. Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H). Transplantation. 2003. 76:120–129.
Article35. Ozdemir BH, Demirhan B, Gungen Y. The presence and prognostic importance of glomerular macrophage infiltration in renal allografts. Nephron. 2002. 90:442–446.
Article36. Karnovsky ML. Metchnikoff in Messina: a century of studies on phagocytosis. N Engl J Med. 1981. 304:1178–1180.37. Rabinovitch M. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 1995. 5:85–87.
Article38. Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998. 188:1359–1368.
Article39. Underhill DM, Bassetti M, Rudensky A, Aderem A. Dynamic interactions of macrophages with T cells during antigen presentation. J Exp Med. 1999. 190:1909–1914.
Article40. Trieb K, Dirnhofer S, Krumbock N, Blahovec H, Sgonc R, Margreiter R, et al. Heat shock protein expression in the transplanted human kidney. Transpl Int. 2001. 14:281–286.
Article41. Breloer M, Moré SH, Osterloh A, Stelter F, Jack RS, Bonin Av A. Macrophages as main inducers of IFN-gamma in T cells following administration of human and mouse heat shock protein 60. Int Immunol. 2002. 14:1247–1253.
Article42. Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998. 392:86–89.
Article43. Teppo AM, Honkanen E, Ahonen J, Grönhagen-Riska C. Does increased urinary interleukin-1 receptor antagonist/interleukin-1beta ratio indicate good prognosis in renal transplant recipients? Transplantation. 1998. 66:1009–1014.
Article44. Kutukculer N, Shenton BK, Clark K, Rigg KM, Forsythe JL, Kirby JA, et al. Renal allograft rejection: the temporal relationship and predictive value of plasma TNF (alpha and beta), IFN-gamma and soluble ICAM-1. Transpl Int. 1995. 8:45–50.
Article45. Wyburn K, Wu H, Yin J, Jose M, Eris J, Chadban S. Macrophage-derived interleukin-18 in experimental renal allograft rejection. Nephrol Dial Transplant. 2005. 20:699–706.
Article46. Halloran PF, Autenried P, Ramassar V, Urmson J, Cockfield S. Local T cell responses induce widespread MHC expression. Evidence that IFN-gamma induces its own expression in remote sites. J Immunol. 1992. 148:3837–3846.47. Ioannidis I, Hellinger A, Dehmlow C, Rauen U, Erhard J, Eigler FW, et al. Evidence for increased nitric oxide production after liver transplantation in humans. Transplantation. 1995. 59:1293–1297.
Article48. Holán V, Krulová M, Zajícová A, Pindjáková J. Nitric oxide as a regulatory and effector molecule in the immune system. Mol Immunol. 2002. 38:989–995.
Article49. Roza AM, Cooper M, Pieper G, Hilton G, Dembny K, Lai CS, et al. NOX 100, a nitric oxide scavenger, enhances cardiac allograft survival and promotes long-term graft acceptance. Transplantation. 2000. 69:227–231.
Article50. Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003. 63:83–95.
Article51. Jose MD, Ikezumi Y, van Rooijen N, Atkins RC, Chadban SJ. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003. 76:1015–1022.
Article52. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003. 349:2326–2333.
Article53. Herrero-Fresneda I, Torras J, Cruzado JM, Condom E, Vidal A, Riera M, et al. Do alloreactivity and prolonged cold ischemia cause different elementary lesions in chronic allograft nephropathy? Am J Pathol. 2003. 162:127–137.
Article54. Joosten SA, van Kooten C, Paul LC. Pathogenesis of chronic allograft rejection. Transpl Int. 2003. 16:137–145.
Article55. Shimizu A, Yamada K, Sachs DH, Colvin RB. Mechanisms of chronic renal allograft rejection. II. Progressive allograft glomerulopathy in miniature swine. Lab Invest. 2002. 82:673–686.
Article56. Azuma H, Nadeau KC, Ishibashi M, Tilney NL. Prevention of functional, structural, and molecular changes of chronic rejection of rat renal allografts by a specific macrophage inhibitor. Transplantation. 1995. 60:1577–1582.
Article57. Yang J, Reutzel-Selke A, Steier C, Jurisch A, Tullius SG, Sawitzki B, et al. Targeting of macrophage activity by adenovirus-mediated intragraft overexpression of TNFRp 55-Ig, IL-12p40, and vIL-10 ameliorates adenovirus-mediated chronic graft injury, whereas stimulation of macrophages by overexpression of IFN-gamma accelerates chronic graft injury in a rat renal allograft model. J Am Soc Nephrol. 2003. 14:214–225.
Article58. Lin Y, Vandeputte M, Waer M. Natural killer cell- and macrophage-mediated rejection of concordant xenografts in the absence of T and B cell responses. J Immunol. 1997. 158:5658–5667.59. Ide K, Ohdan H, Kobayashi T, Hara H, Ishiyama K, Asahara T. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation. 2005. 12:181–188.
Article60. Yi S, Hawthorne WJ, Lehnert AM, Ha H, Wong JK, van Rooijen N, et al. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J Immunol. 2003. 170:2750–2758.
Article61. Abe M, Cheng J, Qi J, Glaser RM, Thall AD, Sykes M, et al. Elimination of porcine hemopoietic cells by macrophages in mice. J Immunol. 2002. 168:621–628.
Article62. Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, et al. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998. 47:316–323.
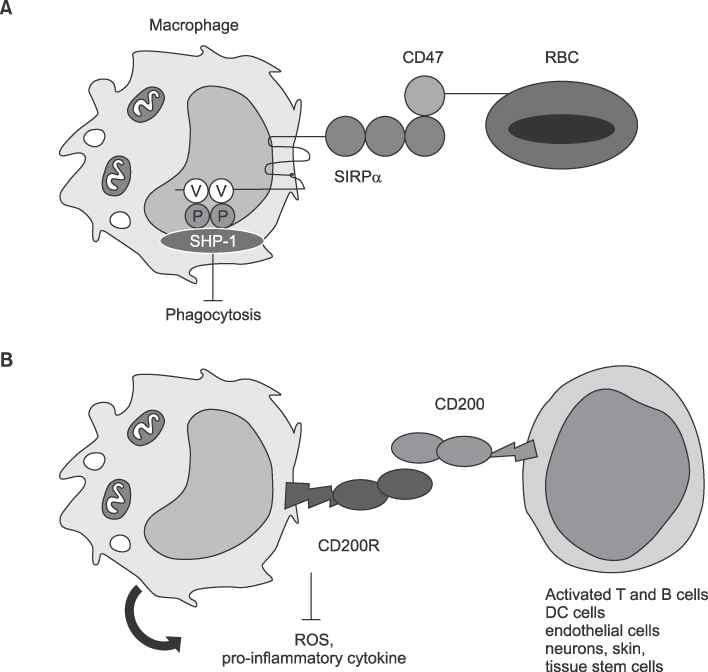
Article63. Wang H, VerHalen J, Madariaga ML, Xiang S, Wang S, Lan P, et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007. 109:836–842.
Article64. Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007. 104:5062–5066.65. Minas K, Liversidge J. Is the CD200/CD200 receptor interaction more than just a myeloid cell inhibitory signal? Crit Rev Immunol. 2006. 26:213–230.
Article66. Gorczynski RM, Lee L, Boudakov I. Augmented Induction of CD4+CD25+ Treg using monoclonal antibodies to CD200R. Transplantation. 2005. 79:1180–1183.
Article67. Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000. 13:233–242.
Article