Korean J Transplant.
2019 Dec;33(4):74-82. 10.4285/jkstn.2019.33.4.74.
Macrophages in xenotransplantation
- Affiliations
-
- 1Department of Life Science, Gachon University, Seongnam, Korea. jykim85@gachon.ac.kr
- KMID: 2468157
- DOI: http://doi.org/10.4285/jkstn.2019.33.4.74
Abstract
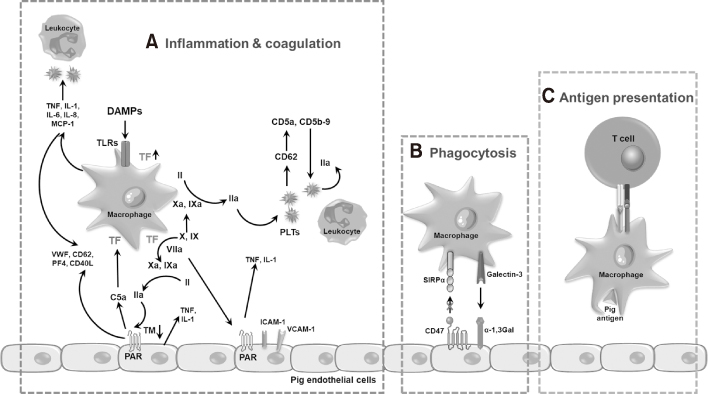
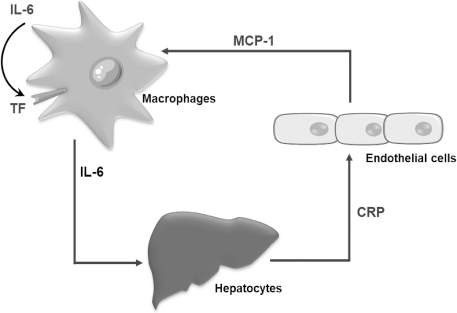
- Xenotransplantation refers to organ transplantation across species. Immune rejection of xenografts is stronger and faster than that of allografts because of significant molecular differences between species. Recent studies have revealed the involvement of macrophages in xenograft and allograft rejections. Macrophages have been shown to play a critical role in inflammation, coagulation, and phagocytosis in xenograft rejection. This review presents a recent understanding of the role of macrophages in xenograft rejection and possible strategies to control macrophage-mediated xenograft rejection.
Keyword
MeSH Terms
Figure
Reference
-
1. Samstein B, Platt JL. Physiologic and immunologic hurdles to xenotransplantation. J Am Soc Nephrol. 2001; 12:182–193.
Article2. Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005; 83:674–686.
Article3. Cooper DK, Ekser B, Tector AJ. Immunobiological barriers to xenotransplantation. Int J Surg. 2015; 23(Pt B):211–216.
Article4. Denner J. Xenotransplantation-progress and problems: a review. J Transplant Technol Res. 2014; 4:1000133.
Article5. Zecher D, van Rooijen N, Rothstein DM, Shlomchik WD, Lakkis FG. An innate response to allogeneic nonself mediated by monocytes. J Immunol. 2009; 183:7810–7816.
Article6. Oberbarnscheidt MH, Zeng Q, Li Q, Dai H, Williams AL, Shlomchik WD, et al. Non-self recognition by monocytes initiates allograft rejection. J Clin Invest. 2014; 124:3579–3589.
Article7. Liu W, Xiao X, Demirci G, Madsen J, Li XC. Innate NK cells and macrophages recognize and reject allogeneic nonself in vivo via different mechanisms. J Immunol. 2012; 188:2703–2711.
Article8. Matheson PJ, Dittmer ID, Beaumont BW, Merrilees MJ, Pilmore HL. The macrophage is the predominant inflammatory cell in renal allograft intimal arteritis. Transplantation. 2005; 79:1658–1662.
Article9. Xu L, Collins J, Drachenberg C, Kukuruga D, Burke A. Increased macrophage density of cardiac allograft biopsies is associated with antibody-mediated rejection and alloantibodies to HLA antigens. Clin Transplant. 2014; 28:554–560.
Article10. Bergler T, Jung B, Bourier F, Kühne L, Banas MC, Rümmele P, et al. Infiltration of macrophages correlates with severity of allograft rejection and outcome in human kidney transplantation. PLoS One. 2016; 11:e0156900.
Article11. Bräsen JH, Khalifa A, Schmitz J, Dai W, Einecke G, Schwarz A, et al. Macrophage density in early surveillance biopsies predicts future renal transplant function. Kidney Int. 2017; 92:479–489.
Article12. Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994; 12:991–1045.
Article13. Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007; 117:2847–2859.
Article14. Kaczorowski DJ, Nakao A, Vallabhaneni R, Mollen KP, Sugimoto R, Kohmoto J, et al. Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation. 2009; 87:1455–1463.
Article15. Yang Z, Deng Y, Su D, Tian J, Gao Y, He Z, et al. TLR4 as receptor for HMGB1-mediated acute lung injury after liver ischemia/reperfusion injury. Lab Invest. 2013; 93:792–800.
Article16. Weber DJ, Gracon AS, Ripsch MS, Fisher AJ, Cheon BM, Pandya PH, et al. The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation. Sci Transl Med. 2014; 6:252ra124.
Article17. Hu Q, Wood CR, Cimen S, Venkatachalam AB, Alwayn IP. Mitochondrial damage-associated molecular patterns (MTDs) are released during hepatic ischemia reperfusion and induce inflammatory responses. PLoS One. 2015; 10:e0140105.
Article18. De Vlaminck I, Martin L, Kertesz M, Patel K, Kowarsky M, Strehl C, et al. Noninvasive monitoring of infection and rejection after lung transplantation. Proc Natl Acad Sci U S A. 2015; 112:13336–13341.
Article19. Gielis EM, Ledeganck KJ, De Winter BY, Del Favero J, Bosmans JL, Claas FH, et al. Cell-free DNA: an upcoming biomarker in transplantation. Am J Transplant. 2015; 15:2541–2551.
Article20. Baba HA, Schmid KW, Schmid C, Blasius S, Heinecke A, Kerber S, et al. Possible relationship between heat shock protein 70, cardiac hemodynamics, and survival in the early period after heart transplantation. Transplantation. 1998; 65:799–804.
Article21. Schimke I, Lutsch G, Schernes U, Kruse I, Dübel HP, Pregla R, et al. Increased level of HSP27 but not of HSP72 in human heart allografts in relation to acute rejection. Transplantation. 2000; 70:1694–1697.
Article22. Sarri S, Shaw SM, Gieschen-Krische MA, Archer L, Yonan N, Fildes JE. Myocardial heat shock protein 60 expression is upregulated following acute cardiac rejection. Transpl Immunol. 2009; 21:140–142.
Article23. Maehana T, Tanaka T, Kitamura H, Fukuzawa N, Ishida H, Harada H, et al. Heat shock protein 90α is a potential serological biomarker of acute rejection after renal transplantation. PLoS One. 2016; 11:e0162942.
Article24. Esmon CT. Molecular circuits in thrombosis and inflammation. Thromb Haemost. 2013; 109:416–420.
Article25. Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014; 509:310–317.
Article26. Yoshida O, Dou L, Kimura S, Yokota S, Isse K, Robson SC, et al. CD39 deficiency in murine liver allografts promotes inflammatory injury and immune-mediated rejection. Transpl Immunol. 2015; 32:76–83.
Article27. Jennewein C, Paulus P, Zacharowski K. Linking inflammation and coagulation: novel drug targets to treat organ ischemia. Curr Opin Anaesthesiol. 2011; 24:375–380.28. Osterud B, Olsen JO, Bjørklid E. What is blood borne tissue factor? Thromb Res. 2009; 124:640–641.
Article29. Ezzelarab M, Garcia B, Azimzadeh A, Sun H, Lin CC, Hara H, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009; 87:805–812.
Article30. Lin CC, Ezzelarab M, Shapiro R, Ekser B, Long C, Hara H, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010; 10:1556–1568.
Article31. Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. 2016; 118:1392–1408.
Article32. Gao H, Liu L, Zhao Y, Hara H, Chen P, Xu J, et al. Human IL-6, IL-17, IL-1β, and TNF-α differently regulate the expression of pro-inflammatory related genes, tissue factor, and swine leukocyte antigen class I in porcine aortic endothelial cells. Xenotransplantation. 2017; 24:e12291.
Article33. Iwase H, Ekser B, Zhou H, Dons EM, Cooper DK, Ezzelarab MB. Platelet aggregation in humans and nonhuman primates: relevance to xenotransplantation. Xenotransplantation. 2012; 19:233–243.
Article34. Ezzelarab MB, Cooper DK. Systemic inflammation in xenograft recipients (SIXR): a new paradigm in pig- to-primate xenotransplantation? Int J Surg. 2015; 23(Pt B):301–305.
Article35. Ezzelarab MB, Ekser B, Azimzadeh A, Lin CC, Zhao Y, Rodriguez R, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015; 22:32–47.
Article36. Iwase H, Ekser B, Zhou H, Liu H, Satyananda V, Humar R, et al. Further evidence for sustained systemic inflammation in xenograft recipients (SIXR). Xenotransplantation. 2015; 22:399–405.
Article37. Dai H, Friday AJ, Abou-Daya KI, Williams AL, Mortin-Toth S, Nicotra ML, et al. Donor SIRPa polymorphism modulates the innate immune response to allogeneic grafts. Sci Immunol. 2017; 2:eaam6202.
Article38. Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007; 104:5062–5066.39. Todd JL, Palmer SM. Danger signals in regulating the immune response to solid organ transplantation. J Clin Invest. 2017; 127:2464–2472.
Article40. Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018; 18:e27.
Article41. Tena A, Kurtz J, Leonard DA, Dobrinsky JR, Terlouw SL, Mtango N, et al. Transgenic expression of human CD47 markedly increases engraftment in a murine model of pig-to-human hematopoietic cell transplantation. Am J Transplant. 2014; 14:2713–2722.
Article42. Hancock WW, Thomson NM, Atkins RC. Composition of interstitial cellular infiltrate identified by monoclonal antibodies in renal biopsies of rejecting human renal allografts. Transplantation. 1983; 35:458–463.
Article43. Candinas D, Lesnikoski BA, Robson SC, Miyatake T, Scesney SM, Marsh HC Jr, et al. Effect of repetitive high-dose treatment with soluble complement receptor type 1 and cobra venom factor on discordant xenograft survival. Transplantation. 1996; 62:336–342.
Article44. Lin Y, Vandeputte M, Waer M. Natural killer cell- and macrophage-mediated rejection of concordant xenografts in the absence of T and B cell responses. J Immunol. 1997; 158:5658–5667.45. Samy KP, Davis RP, Gao Q, Martin BM, Song M, Cano J, et al. Early barriers to neonatal porcine islet engraftment in a dual transplant model. Am J Transplant. 2018; 18:998–1006.
Article46. Ehrnfelt C, Kumagai-Braesch M, Uzunel M, Holgersson J. Adult porcine islets produce MCP-1 and recruit human monocytes in vitro. Xenotransplantation. 2004; 11:184–194.
Article47. An HJ, Jang JW, Bae SH, Choi JY, Yoon SK, Lee MA, et al. Serum C-reactive protein is a useful biomarker for predicting outcomes after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2012; 18:1406–1414.
Article48. Boras E, Slevin M, Alexander MY, Aljohi A, Gilmore W, Ashworth J, et al. Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine. 2014; 69:165–179.
Article49. Han KH, Hong KH, Park JH, Ko J, Kang DH, Choi KJ, et al. C-reactive protein promotes monocyte chemoattractant protein-1—mediated chemotaxis through upregulating CC chemokine receptor 2 expression in human monocytes. Circulation. 2004; 109:2566–2571.
Article50. Kruithof EK, Mestries JC, Gascon MP, Ythier A. The coagulation and fibrinolytic responses of baboons after in vivo thrombin generation: effect of interleukin 6. Thromb Haemost. 1997; 77:905–910.51. Hage FG, Szalai AJ. C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol. 2007; 50:1115–1122.
Article52. Li J, Hara H, Wang Y, Esmon C, Cooper DK, Iwase H. Evidence for the important role of inflammation in xenotransplantation. J Inflamm (Lond). 2019; 16:10.
Article53. Cooper DK, Dou KF, Tao KS, Yang ZX, Tector AJ, Ekser B. Pig liver xenotransplantation: a review of progress toward the clinic. Transplantation. 2016; 100:2039–2047.54. Engel D, Seijkens T, Poggi M, Sanati M, Thevissen L, Beckers L, et al. The immunobiology of CD154-CD40-TRAF interactions in atherosclerosis. Semin Immunol. 2009; 21:308–312.
Article55. Li T, Lee W, Hara H, Long C, Ezzelarab M, Ayares D, et al. An investigation of extracellular histones in pig-to-baboon organ xenotransplantation. Transplantation. 2017; 101:2330–2339.
Article56. Iwase H, Liu H, Li T, Zhang Z, Gao B, Hara H, et al. Therapeutic regulation of systemic inflammation in xenograft recipients. Xenotransplantation. 2017; 24:e12296.
Article57. Chung H, Hong SJ, Choi SW, Koo JY, Kim M, Kim HJ, et al. High mobility group box 1 secretion blockade results in the reduction of early pancreatic islet graft loss. Biochem Biophys Res Commun. 2019; 514:1081–1086.
Article58. Vergani A, Tezza S, D'Addio F, Fotino C, Liu K, Niewczas M, et al. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation. 2013; 127:463–475.
Article59. Wu H, Steenstra R, de Boer EC, Zhao CY, Ma J, van der Stelt JM, et al. Preconditioning with recombinant high-mobility group box 1 protein protects the kidney against ischemia-reperfusion injury in mice. Kidney Int. 2014; 85:824–832.
Article60. Izuishi K, Tsung A, Jeyabalan G, Critchlow ND, Li J, Tracey KJ, et al. Cutting edge: high-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J Immunol. 2006; 176:7154–7158.
Article61. Seemampillai B, Germack R, Felkin LE, McCormack A, Rose ML. Heat shock protein-27 delays acute rejection after cardiac transplantation: an experimental model. Transplantation. 2014; 98:29–38.
Article62. Qi F, Adair A, Ferenbach D, Vass DG, Mylonas KJ, Kipari T, et al. Depletion of cells of monocyte lineage prevents loss of renal microvasculature in murine kidney transplantation. Transplantation. 2008; 86:1267–1274.
Article63. Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, et al. Cutting edge: immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011; 187:2072–2078.
Article64. Riquelme P, Haarer J, Kammler A, Walter L, Tomiuk S, Ahrens N, et al. TIGIT(+) iTregs elicited by human regulatory macrophages control T cell immunity. Nat Commun. 2018; 9:2858.
Article65. Hutchinson JA, Brem-Exner BG, Riquelme P, Roelen D, Schulze M, Ivens K, et al. A cell-based approach to the minimization of immunosuppression in renal transplantation. Transpl Int. 2008; 21:742–754.
Article66. Netea MG, Latz E, Mills KH, O'Neill LA. Innate immune memory: a paradigm shift in understanding host defense. Nat Immunol. 2015; 16:675–679.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulatory macrophages in solid organ xenotransplantation
- Regulatory macrophages as potential cell-based immunotherapy for organ xenotransplantation
- Current Status of Solid Organ Xenotransplantation
- Implications of Calcineurin/NFAT Inhibitors' Regulation of Dendritic Cells and Innate Immune Cells in Islet Xenotransplantation
- Overcoming Immunological Barriers in Xenotransplantation: A Historical Review of Previous Research and Future Directions