J Adv Prosthodont.
2010 Mar;2(1):7-13. 10.4047/jap.2010.2.1.7.
Angiogenic factor-enriched platelet-rich plasma enhances in vivo bone formation around alloplastic graft material
- Affiliations
-
- 1Department of Oral & Maxillofacial Surgery, College of Medicine, Chungnam National University, Daejeon, Korea.
- 2Division of Prosthodontics, School of Medicine, Ewha Womans University, Seoul, Korea. prosth@ewha.ac.kr
- KMID: 1975171
- DOI: http://doi.org/10.4047/jap.2010.2.1.7
Abstract
- PURPOSE
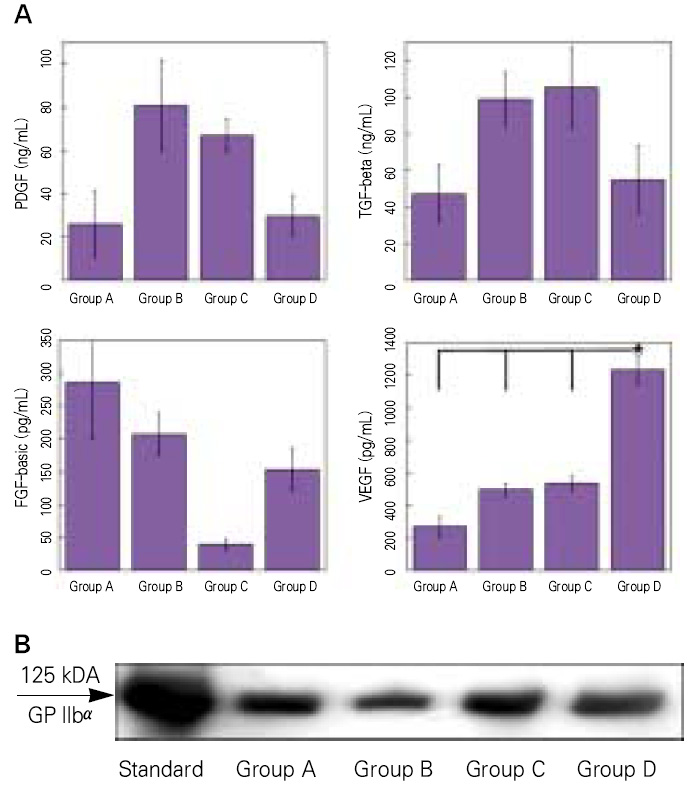
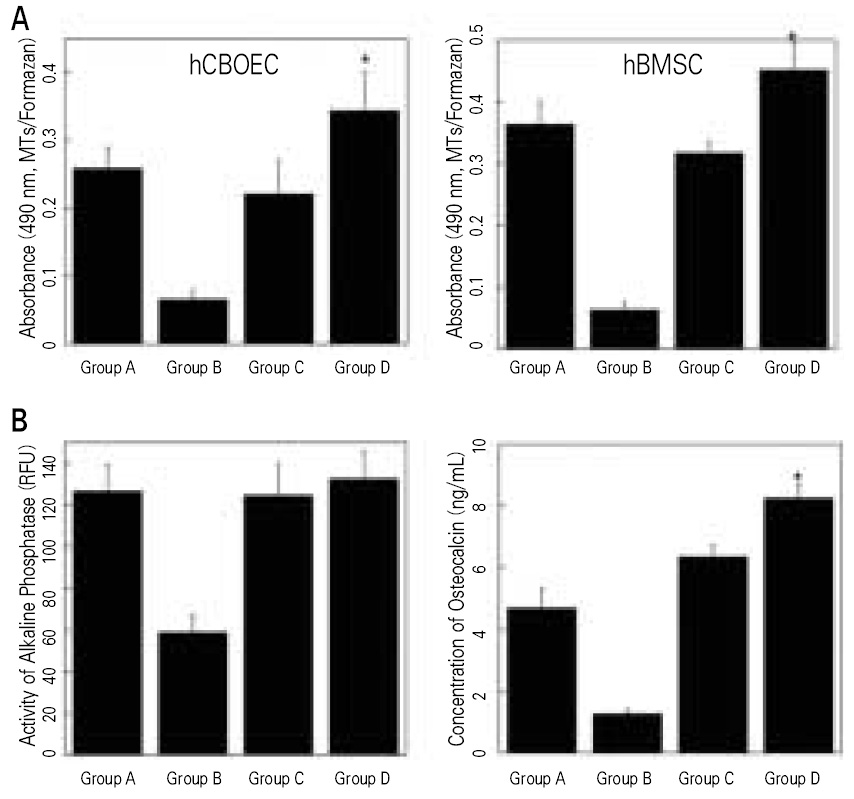
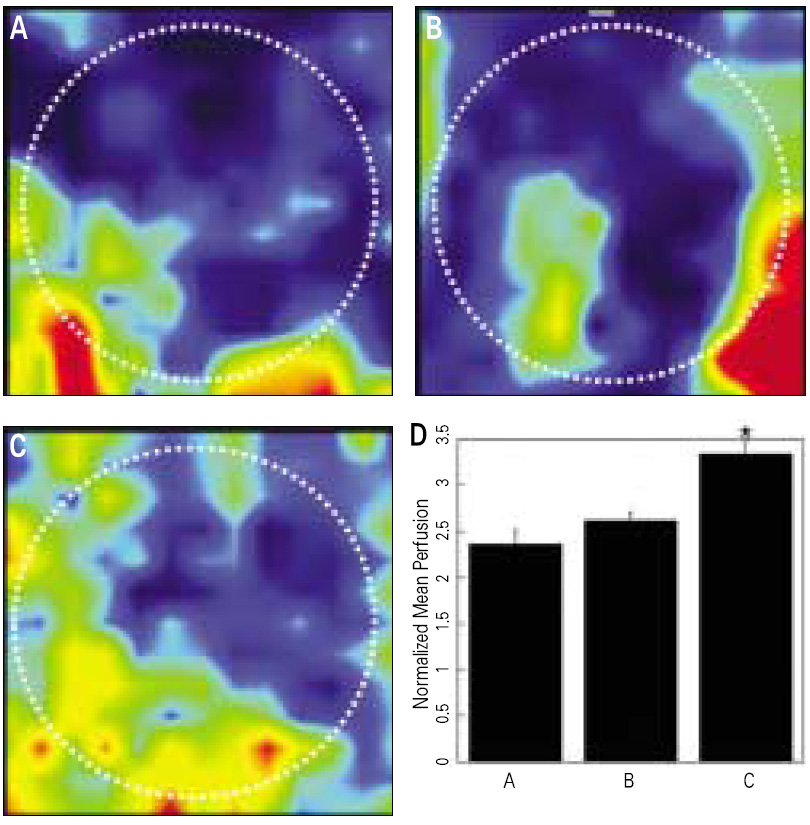
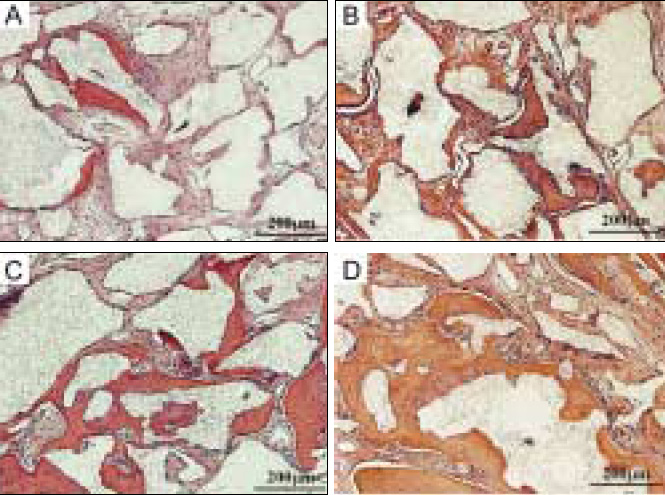
Although most researchers agree that platelet-rich plasma (PRP) is a good source of autogenous growth factors, its effect on bone regeneration is still controversial. The purpose of this study was to evaluate whether increasing angiogenic factors in the human PRP to enhance new bone formation through rapid angiogenesis. MATERIAL AND METHODS: In vitro, the human platelets were activated with application of shear stress, 20 microgram/ml collagen, 2 mM CaCl2 and 10U thrombin/1 x 109 platelets. Level of vascular endothelial growth factor (VEGF) and platelet microparticle (PMP) in the activated platelets were checked. In the animal study, human angiogenic factors-enriched PRP was tested in 28 athymic rat's cranial critical bone defects with beta-TCP. Angiogenesis and osteogenesis were evaluated by laser Doppler perfusion imaging, histology, dual energy X-ray densinometry, and micro-computed tomography.
RESULTS
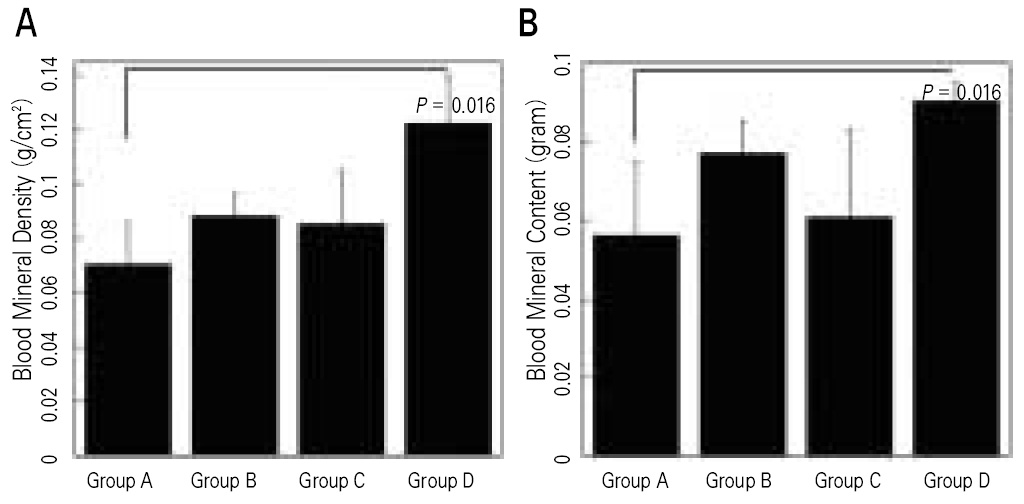
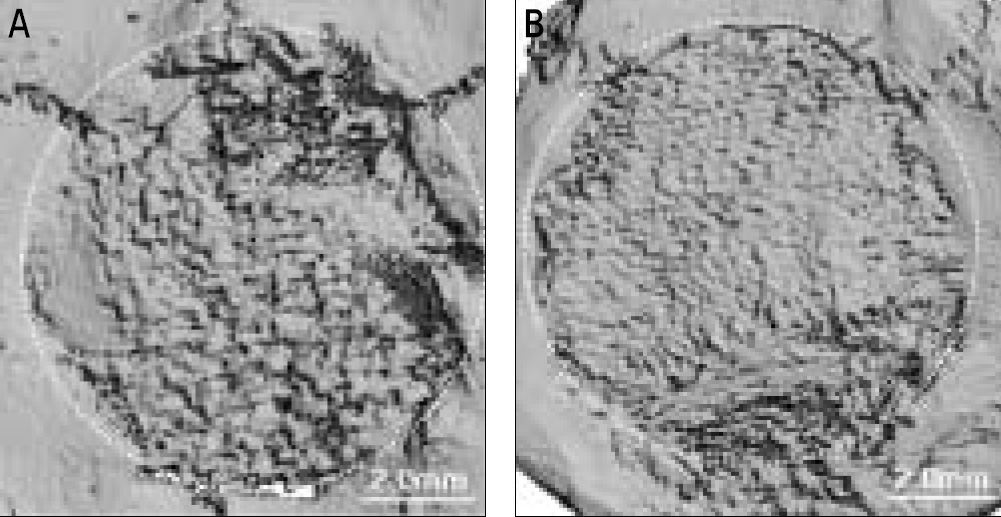
In vitro, this human angiogenic factors-enriched PRP resulted in better cellular proliferation and osteogenic differentiation. In vivo, increasing angiogenic potential of the PRP showed significantly higher blood perfusion around the defect and enhanced new bone formation around acellular bone graft material.
CONCLUSION
Angiogenic factor-enriched PRP leads to faster and more extensive new bone formation in the critical size bone defect. The results implicate that rapid angiogenesis in the initial healing period by PRP could be supposed as a way to overcome short term effect of the rapid angiogenesis.
Keyword
MeSH Terms
-
Angiogenesis Inducing Agents
Animals
Blood Platelets
Bone Regeneration
Calcium Phosphates
Cell Proliferation
Collagen
Durapatite
Humans
Intercellular Signaling Peptides and Proteins
Osteogenesis
Perfusion
Perfusion Imaging
Platelet-Rich Plasma
Rats, Nude
Transplants
Vascular Endothelial Growth Factor A
Angiogenesis Inducing Agents
Calcium Phosphates
Collagen
Durapatite
Intercellular Signaling Peptides and Proteins
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:638–646.2. Butterfield KJ, Bennett J, Gronowicz G, Adams D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg. 2005. 63:370–376.3. Pryor ME, Polimeni G, Koo KT, Hartman MJ, Gross H, April M, Safadi FF, Wikesjö UM. Analysis of rat calvaria defects implanted with a platelet-rich plasma preparation: histologic and histometric observations. J Clin Periodontol. 2005. 32:966–972.4. Raghoebar GM, Schortinghuis J, Liem RS, Ruben JL, van der Wal JE, Vissink A. Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005. 16:349–356.5. Fürst G, Gruber R, Tangl S, Zechner W, Haas R, Mailath G, Sanroman F, Watzek G. Sinus grafting with autogenous platelet-rich plasma and bovine hydroxyapatite. A histomorphometric study in minipigs. Clin Oral Implants Res. 2003. 14:500–508.6. Grageda E. Platelet-rich plasma and bone graft materials: a review and a standardized research protocol. Implant Dent. 2004. 13:301–309.7. Suba Z, Takacs D, Gyulai-Gaal S, Kovacs K. Facilitation of beta-tricalcium phosphate-induced alveolar bone regeneration by platelet-rich plasma in beagle dogs: a histologic and histomorphometric study. Int J Oral Maxillofac Implants. 2004. 19:832–838.8. Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005. 20:848–857.9. Chow KM, Rabie AB. Vascular endothelial growth pattern of endochondral bone graft in the presence of demineralized intramembranous bone matrix--quantitative analysis. Cleft Palate Craniofac J. 2000. 37:385–394.10. Rabie AB, Chow KM, Wong RW. Demineralized intramembranous bone matrix augments the healing of endochondral bone graft. Int J Adult Orthodon Orthognath Surg. 1999. 14:185–197.11. Street J, Bao M, deGuzman L, Bunting S, Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002. 99:9656–9661.12. Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Günther KP, Dehio C, Puhl W, Brenner RE. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002. 30:472–477.13. Schlegel KA, Zimmermann R, Thorwarth M, Neukam FW, Klongnoi B, Nkenke E, Felszeghy E. Sinus floor elevation using autogenous bone or bone substitute combined with platelet-rich plasma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007. 104:e15–e25.14. Borrelli V, Sterpetti AV, Coluccia P, Randone B, Cavallaro A, Santoro D' Angelo L, Cucina A. Bimodal concentration-dependent effect of thrombin on endothelial cell proliferation and growth factor release in culture. J Surg Res. 2001. 100:154–160.15. Karp JM, Tanaka TS, Zohar R, Sodek J, Shoichet MS, Davies JE, Stanford WL. Thrombin mediated migration of osteogenic cells. Bone. 2005. 37:337–348.16. Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999. 103:159–165.17. Horstman LL, Ahn YS. Platelet microparticles: a wide-angle perspective. Crit Rev Oncol Hematol. 1999. 30:111–142.18. Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004. 124:376–384.19. Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, Kojima H, Iwasaka T. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002. 106:2019–2025.20. Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, Komiyama Y, Fujimura Y, Ikeda Y, Fukuhara S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996. 88:3456–3464.21. Holme PA, Solum NO, Brosstad F, Roger M, Abdelnoor M. Demonstration of platelet-derived microvesicles in blood from patients with activated coagulation and fibrinolysis using a filtration technique and western blotting. Thromb Haemost. 1994. 72:666–671.22. Park EJ, Kim ES, Weber H-P, Wright RF, Mooney DJ. Improved bone healing by angiogenic-facotr enriched platelet-rich plasma and its synergistic enhancement by bone morphogenic protein-2. Int J Oral Maxillofac Impl. 2008. 23:818–826.23. Roussy Y, Bertrand Duchesne MP, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res. 2007. 18:639–648.24. Martineau I, Lacoste E, Gagnon G. Effect of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004. 25:4489–4502.25. Maloney JP, Sillimann CC, Ambruso DR, Wang J, Tuder RM, Voelkel NF. In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Physiol. 1998. 275:H1054–H1061.26. Gruber R, Karreth F, Kandler B, Fuerst G, Rot A, Fischer MB, Watzek G. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets. 2004. 15:29–35.27. Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005. 19:665–667.28. Kowalski MJ, Schemitsch EH, Kregor PJ, Senft D, Swiontkowski MF. Effect of periosteal stripping on cortical bone perfusion: a laser Doppler study in sheep. Calcif Tissue Int. 1996. 59:24–26.29. Zammaretti P, Zisch AH. Adult 'endothelial progenitor cells'. Renewing vasculature. Int J Biochem Cell Biol. 2005. 37:493–503.30. Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002. 283:H2411–H2419.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison on New Bone Formation Between Ovariectomized Rats and Normal Rats After Graft of Alloplastic bone material
- Use of Platelet-rich Plasma
- Effect of bovine bone (Bio-Oss(R)) and platelet rich plasma, platelet poor plasma on sinus bone graft in rabbit
- Effect of platelet-rich plasma on bone regeneration in ovariectomized osteoporotic rats
- The Regenerative effects of Platelet-Rich Plasma and Enamel Matrix Protein on Grade III Furcation defects in beagle dogs