Tuberc Respir Dis.
2007 Aug;63(2):165-172. 10.4046/trd.2007.63.2.165.
Comparison of Immunohistochemical Expression of CBP(cAMP- responsive Element Binding Protein) Transcriptional Co-activator between Premalignant Lesions and Squamous Cell Carcinomas in the Lungs
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Chung-Ang University, Seoul, Korea.
- 2Department of Pathology, College of Medicine, Chung-Ang University, Seoul, Korea. ladymkk603@yahoo.co.kr
- KMID: 1910095
- DOI: http://doi.org/10.4046/trd.2007.63.2.165
Abstract
-
BACKGROUND: The pathogenesis of lung cancer includes the accumulation of multiple genetic abnormalities. The CREB-binding protein(CBP) is one of several transcriptional co-activators among various sequence-specific DNA-binding transcription factors. CBP is involved in a wide range of cellular activities, such as DNA repair, cell growth, differentiation, and apoptosis that are suspected of contributing to tumorigenesis. The goal of this study was to evaluate CBP expression in a series of human lung tissues containing normal epithelium, premalignant lesions(hyperplasia and dysplasia) and squamous cell carcinomas.
MATERIALS AND METHODS
Immunohistochemical staining was performed on formalin-fixed paraffin-embedded sections by use of a monoclonal anti-CBP antibody. CBP expression was compared in samples from 120 patients with premalignant and malignant histological types including 20 metaplastic specimens, 40 dysplastic specimens, and 60 squamous cell carcinomas in the lung.
RESULTS
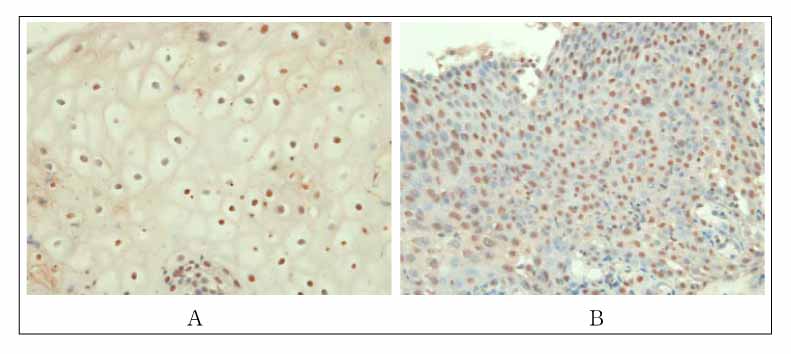
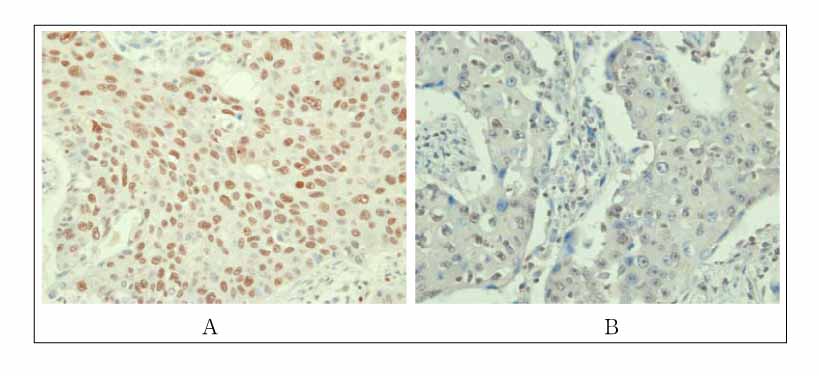
CBP expression was seen in 35% (7/20) of the metaplastic specimens. 65% (26/40) of the dysplastic specimens, and 70% (42/60) of the squamous cell carcinomas (p<0.05). According to celluar atypism, CBP expression was 50% (10/20) of the low-grade dysplastic specimens and 80% (16/20) of the high-grade dysplastic specimens(p <0.01). By cellular differentiation, CBP expression was seen in 95% (19/20) of the well differentiated squamous cell carcinomas, 85% (17/20) of the moderately differentiated carcinomas and 30% (6/20) of the poorly differentiated lesions (p <0.05).
CONCLUSION
These results suggest that CBP may have an important role in malignant transformation of precancerous lung lesions and may be a marker for malignancy.
Keyword
MeSH Terms
Figure
Reference
-
1. Gazdar AF, Minna JD. Pas HI, Carbone DP, Minna JD, Johnson DH, Turrisi AT, editors. Chapter 16, Molecular techniques of early detection of lung cancer and for studying preneoplasia. Lung cancer: principles and practice. 2005. Philadelphia, PA: Lippincott Williams & Wilkins;200–209.2. Haber DA, Fearon ER. The promise of cancer genetics. Lancet. 1998. 351:SII1–SII8.3. Harris AL. Antiangiogenesis for cancer therapy. Lancet. 1997. 349:SII13–SII15.4. Levitzki A. Targeting signal transduction for disease therapy. Curr Opin Cell Biol. 1996. 8:239–244.5. Fearon ER. Human cancer syndromes: clues to the origin and nature of cancer. Science. 1997. 278:1043–1050.6. Karin M, Smeal T. Control of transcription factors by signal transduction pathways: the beginning of the end. Trends Biochem Sci. 1992. 17:418–422.7. Quinn PG. Distinct activation domains within cAMP response element-binding protein(CREB) mediate basal and cAMP-stinulated transcription. J Biol Chem. 1993. 268:16999–17009.8. Sassone-Corsi P. Transcription factors responsive to cAMP. Annu Rev Cell Dev Biol. 1995. 11:355–377.9. Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001. 276:13505–13508.10. Xu L, Lavinsky RM, Dasen JS, Flynn S, McInerney EM, Mullen TM, et al. Signal-specific co-activator domain requirements for Pit-1 activation. Nature. 1998. 395:301–306.11. Giordano A, Avantaggiati ML. p300 and CBP: partners for life and death. J Cell Physiol. 1999. 181:218–230.12. Grossman SR. p300/CBP/p53 interaction and regulation of the p53 response. Eur J Biochem. 2001. 268:2773–2778.13. Ito A, Lai CH, Zhao X, Saito S, Hamilton MH, Appella E, et al. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 2001. 20:1331–1340.14. Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997. 90:595–606.15. Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997. 80:1175–1184.16. Gu W, Shi XL, Roeder RG. Synergistic activation of transcription by CBP and p53. Nature. 1997. 387:819–823.17. Lill NL, Grossman SR, Ginsberg D, DeCaprio J, Livingston DM. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997. 387:823–827.18. Pao GM, Janknecht R, Ruffner H, Hunter T, Verma IM. CBP/p300 interact with and function as transcriptional coactivators of BRCA1. Proc Natl Acad Sci U S A. 2000. 97:1020–1025.19. Borrow J, Stanton VP Jr, Andresen JM, Becher R, Behm FG, Chaganti RS, et al. The translocation t(8;16(p11;p13)) of acute myelogenous leukemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996. 14:33–41.20. Rowley JD, Reshmi S, Sobulo O, Musvee T, Anastasi J, Raimondi S, et al. All patients with the T(11;16)(q23;p13.3) that involves MLL and CBP have treatment-related hematologic disorders. Blood. 1997. 90:535–541.21. Sobulo OM, Borrow J, Tomek R, Reshmi S, Harden A, Schlegelberger B, et al. MLL is fused to CBP, a histoneacetyl transferase, intherapy-related acute myeloid leukemia with a t(11;16)(q23;p13.3). Proc Natl Acad Sci U S A. 1997. 94:8732–8737.22. Kitabayashi I, Yokoyama A, Shimizu K, Ohki M. Interaction and functional cooperation of the leukemia-associated factors AML1 and p300 in myeloid cell differentiation. EMBO J. 1998. 17:2994–3004.23. Huang S, Qiu Y, Stein RW, Brandt SJ. p300 functions as a transcriptional coactivator for the TAL1/SCL oncoprotein. Oncogene. 1999. 18:4958–4967.24. Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M, et al. Activation of cAMP an mitogen responsive genes relies on a common nuclear factor. Nature. 1994. 370:226–229.25. Bannister AJ, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995. 11:2509–2514.26. Bannister AJ, Kouzarides T. CBP-induced stimulation of c-Fos activity is abrogated by E1A. EMBO J. 1995. 14:4758–4762.27. Dai P, Akimaru H, Tanaka Y, Hou DX, Yasukawa T, Kanei-Ishii C, et al. CBP as a transcriptional coactivator of c-Myb. Genes Dev. 1996. 10:528–540.28. Trouche D, Cook A, Kouzarides T. The CBP co-activator stimulates E2F1/DP1 activity. Nucleic Acids Res. 1996. 24:4139–4145.29. Krecicki T, Jelen M, Zalesska-krecicka M, Szkudlarek T, Szajowski K. Immunohistochemically stained markers (p53, PCNA, bcl-2) in dysplastic lesions of the larynx. Cancer Lett. 1999. 143:23–28.30. Karamouzis MV, Papadas T, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Induction of the CBP transcriptional co-activator early during laryngeal carcinogenesis. J Cancer Res Clin Oncol. 2002. 128:135–140.31. Goodman RH, Smolik S. CBP/p300 in cell growth, transformation and development. Genes Dev. 2000. 14:1553–1577.32. Kishimoto M, Kohno T, Okudela K, Otsuka A, Sasaki H, Tanabe C, et al. Mutations and deletions of the CBP gene in human lung cancer. Clin Cancer Res. 2005. 11:512–519.33. Adcock IM, Ito K, Barnes PJ. Histone deacetylation: an important mechanism in inflammatory lung diseases. COPD. 2005. 2:445–455.34. Huang WC, Ju TK, Hung MC, Chen CC. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol Cell. 2007. 26:75–87.35. Gorgoulis VG, Zacharatos P, Mariatos G, Kotsinas A, Bouda M, Kletsas D, et al. Transcription factor E2F-1 acts as a growth-promoting factor and is associated with adverse prognosis in non-small cell lung carcinomas. J Pathol. 2002. 198:142–156.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Expression of Nuclear Retinoid Receptor and CREB(cAMP Response Element Binding Protein) in Lung Cancers
- β-Catenin Regulates the Expression of cAMP Response Element-Binding Protein 1 in Squamous Cell Carcinoma Cells
- Immunohistochemical localization of cyclic AMP-responsive element binding protein (CREB)-binding protein in the pig retina during postnatal development
- Differential Roles of Transcriptional Coactivators: CBP and CIITA on GAS (Interferon-r Activated Site) - Mediated Transcription in Thyroid Cells
- Immunohistochemical Expression of p53 Protein and CREB-binding Protein in Polyps and Adenocarcinomas of Colon