Nutr Res Pract.
2013 Oct;7(5):366-372. 10.4162/nrp.2013.7.5.366.
Effect of green tea extract microencapsulation on hypertriglyceridemia and cardiovascular tissues in high fructose-fed rats
- Affiliations
-
- 1Department of Food and Nutrition, Kookmin University, 861-1 Chongneung-dong, Sungbuk-gu, Seoul 136-702, Korea. cmoon@kookmin.ac.kr
- 2National Institute of Animal Science, Rural Development Administration, Suwon, Gyeonggi 441-707, Korea.
- 3Department of Food Engineering, Dankook University, Cheonan, Chungnam 330-714, Korea.
- 4Department of Pathology, College of Medicine, Dankook University, Cheonan, Chungnam 330-714, Korea.
- KMID: 1841307
- DOI: http://doi.org/10.4162/nrp.2013.7.5.366
Abstract
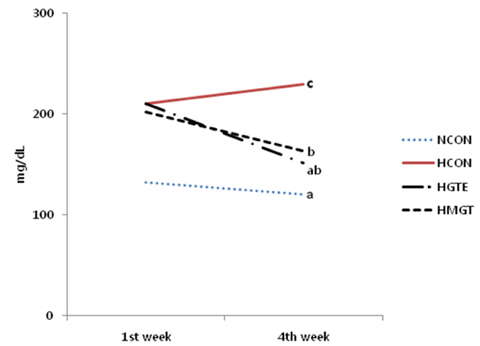
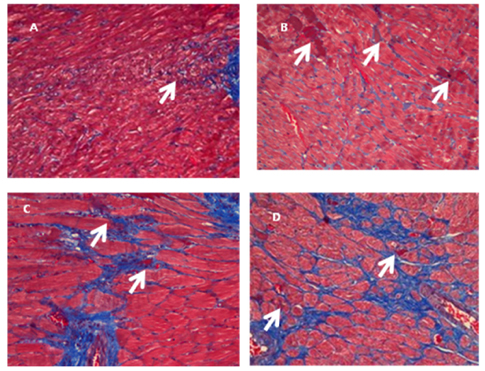
- The application of polyphenols has attracted great interest in the field of functional foods and nutraceuticals due to their potential health benefits in humans. However, the effectiveness of polyphenols depends on their bioactivity and bioavailability. In the present study, the bioactive component from green tea extract (GTE) was administrated orally (50 mg/kg body weight/day) as free or in a microencapsulated form with maltodextrin in rats fed a high fructose diet. High fructose diet induced features of metabolic syndrome including hypertriglyceridemia, hyperuricemia, increased serum total cholesterol, and retroperitoneal obesity. In addition, myocardial fibrosis was increased. In rats receiving high fructose diet, the lowering of blood triglycerides, total cholesterol, non esterified fatty acid (NEFA) and uric acid, as well as the reduction in final body weight and retroperitoneal fat weight associated with the administration of GTE, led to a reversal of the features of metabolic syndrome (P < 0.05). In particular, the administration of microencapsulated GTE decreased myocardial fibrosis and increased liver catalase activity consistent with a further alleviation of serum NEFA, and hyperuricemia compared to administration of GTE. Taken together, our results suggest that microencapsulation of the bioactive components of GTE might have a protective effect on cardiovasucular system by attenuating the adverse features of myocardial fibrosis, decreasing uric acid levels and increasing hepatic catalase activity effectively by protecting their bioactivities.
MeSH Terms
-
Animals
Biological Availability
Body Weight
Catalase
Cholesterol
Diet
Dietary Supplements
Drug Compounding
Fatty Acids, Nonesterified
Fibrosis
Fructose
Functional Food
Humans
Hypertriglyceridemia
Hyperuricemia
Insurance Benefits
Intra-Abdominal Fat
Liver
Obesity
Polyphenols
Polysaccharides
Rats
Tea
Triglycerides
Uric Acid
Catalase
Cholesterol
Fatty Acids, Nonesterified
Fructose
Polyphenols
Polysaccharides
Tea
Triglycerides
Uric Acid
Figure
Reference
-
1. Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001; 24:683–689.
Article2. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003; 163:427–436.3. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106:3143–3421.4. Grundy SM, Hansen B, Smith SC Jr, Cleeman JI, Kahn RA. American Heart Association. National Heart, Lung, and Blood Institute. American Diabetes Association. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol. 2004; 24:e19–e24.5. Austin MA. Plasma triglyceride and coronary heart disease. Arterioscler Thromb. 1991; 11:2–14.
Article6. Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998; 81:7B–12B.
Article7. Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996; 276:882–888.
Article8. Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Després JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997; 95:69–75.
Article9. Shankar A, Mitchell P, Rochtchina E, Wang JJ. The association between circulating white blood cell count, triglyceride level and cardiovascular and all-cause mortality: population-based cohort study. Atherosclerosis. 2007; 192:177–183.
Article10. Dauchet L, Montaye M, Ruidavets JB, Arveiler D, Kee F, Bingham A, Ferrières J, Haas B, Evans A, Ducimetière P, Amouyel P, Dallongeville J. Association between the frequency of fruit and vegetable consumption and cardiovascular disease in male smokers and non-smokers. Eur J Clin Nutr. 2010; 64:578–586.
Article11. Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol. 2004; 160:1223–1233.
Article12. Greenlee H. Natural products for cancer prevention. Semin Oncol Nurs. 2012; 28:29–44.
Article13. Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003; 133:3275S–3284S.
Article14. Ebrahimzadeh MA, Nabavi SM, Nabavi SF. Correlation between the in vitro iron chelating activity and poly phenol and flavonoid contents of some medicinal plants. Pak J Biol Sci. 2009; 12:934–938.
Article15. Tuñón MJ, García-Mediavilla MV, Sánchez-Campos S, González-Gallego J. Potential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathways. Curr Drug Metab. 2009; 10:256–271.16. Yue X, Xu Z. Changes of anthocyanins, anthocyanidins, and antioxidant activity in bilberry extract during dry heating. J Food Sci. 2008; 73:C494–C499.
Article17. Kumamoto M, Sonda T, Nagayama K, Tabata M. Effects of pH and metal ions on antioxidative activities of catechins. Biosci Biotechnol Biochem. 2001; 65:126–132.
Article18. Chen Z, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. J Agric Food Chem. 2001; 49:477–482.
Article19. Rodriguez-Saona LE, Giusti MM, Wrolstad RE. Color and pigment stability of red radish and red-fleshed potato anthocyanins in juice model systems. J Food Sci. 1999; 64:451–456.
Article20. Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: vaccinium, rubus, and ribes. J Agric Food Chem. 2002; 50:519–525.
Article21. Cai YZ, Corke H. Production and properties of spray-dried amaranthus betacyanin pigments. J Food Sci. 2000; 65:1248–1252.
Article22. Ersus S, Yurdagel U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J Food Eng. 2007; 80:805–812.
Article23. Augustin MA, Hemar Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem Soc Rev. 2009; 38:902–912.
Article24. Augustin MA, Abeywardena MY, Patten G, Head R, Lockett T, De Luca A, Sanguansri L. Effects of microencapsulation on the gastrointestinal transit and tissue distribution of a bioactive mixture of fish oil, tributyrin and resveratrol. J Funct Foods. 2011; 3:25–37.
Article25. Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006; 290:F625–F631.
Article26. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951.
Article27. Ueno T, Tremblay J, Kunes J, Zicha J, Dobesova Z, Pausova Z, Deng AY, Sun YL, Jacob HJ, Hamet P. Rat model of familial combined hyperlipidemia as a result of comparative mapping. Physiol Genomics. 2004; 17:38–47.
Article28. Zheng G, Sayama K, Okubo T, Juneja LR, Oguni I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo. 2004; 18:55–62.29. Lin JK, Lin-Shiau SY. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol Nutr Food Res. 2006; 50:211–217.
Article30. Wise EM Jr, Ball EG. Malic enzyme and lipogenesis. Proc Natl Acad Sci U S A. 1964; 52:1255–1263.
Article31. Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952; 195:133–140.
Article32. Busserolles J, Gueux E, Rock E, Demigné C, Mazur A, Rayssiguier Y. Oligofructose protects against the hypertriglyceridemic and pro-oxidative effects of a high fructose diet in rats. J Nutr. 2003; 133:1903–1908.
Article33. Hininger-Favier I, Benaraba R, Coves S, Anderson RA, Roussel AM. Green tea extract decreases oxidative stress and improves insulin sensitivity in an animal model of insulin resistance, the fructose-fed rat. J Am Coll Nutr. 2009; 28:355–361.
Article34. Thresher JS, Podolin DA, Wei Y, Mazzeo RS, Pagliassotti MJ. Comparison of the effects of sucrose and fructose on insulin action and glucose tolerance. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R1334–R1340.
Article35. Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr. 2000; 72:1128–1134.
Article36. Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993; 58:754S–765S.
Article37. Busserolles J, Rock E, Gueux E, Mazur A, Grolier P, Rayssiguier Y. Short-term consumption of a high-sucrose diet has a pro-oxidant effect in rats. Br J Nutr. 2002; 87:337–342.
Article38. Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG, Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab. 2003; 284:E863–E873.39. Yang LY, Kuksis A, Myher JJ, Steiner G. Contribution of de novo fatty acid synthesis to very low density lipoprotein triacylglycerols: evidence from mass isotopomer distribution analysis of fatty acids synthesized from [2H6]ethanol. J Lipid Res. 1996; 37:262–274.
Article40. Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005; 67:1739–1742.
Article41. Hinglais N, Heudes D, Nicoletti A, Mandet C, Laurent M, Bariéty J, Michel JB. Colocalization of myocardial fibrosis and inflammatory cells in rats. Lab Invest. 1994; 70:286–294.42. Thakker GD, Frangogiannis NG, Bujak M, Zymek P, Gaubatz JW, Reddy AK, Taffet G, Michael LH, Entman ML, Ballantyne CM. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006; 291:H2504–H2514.
Article43. Jun YK, Kim MH, Seong PN, Chang MJ. A comparison of anti-inflammatory activities of green tea and grapefruit seed extract with those of microencapsulated extracts. Korean J Nutr. 2012; 45:443–451.
Article44. Yanagi S, Matsumura K, Marui A, Morishima M, Hyon SH, Ikeda T, Sakata R. Oral pretreatment with a green tea polyphenol for cardioprotection against ischemia-reperfusion injury in an isolated rat heart model. J Thorac Cardiovasc Surg. 2011; 141:511–517.
Article45. Bell LN. Stability testing of nutraceuticals and functional foods. In : Wildman RE, editor. Handbook of Nutraceuticals and Functional Foods. Boca Raton (FL): CRC Press;2001. p. 501–516.46. Lambert JD, Kwon SJ, Hong J, Yang CS. Salivary hydrogen peroxide produced by holding or chewing green tea in the oral cavity. Free Radic Res. 2007; 41:850–853.
Article47. Champagne CP, Fustier P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr Opin Biotechnol. 2007; 18:184–190.
Article48. Lee HD, Kim HS, Seong PN, Kim MH. Effect of microencapsulation procession on functional property of natural pigment and antioxidant. Food Eng Prog. 2012; 16:283–286.49. Reineccius GA, Ward FM, Whorton C, Andon SA. Developments in gum acacias for the encapsulation of flavors. In : Risch SJ, Reineccius GA, editors. Encapsulation and Controlled Release of Food Ingredients. Washington, D.C.: ACS Publications;1995. p. 161–168.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Green Tea on Weight Gain, Plasma and Liver Lipids and Lipid Peroxidation in Pair Fed Rats
- Effect of Drinking Green Tea on Glycation and Oxidation of Aortic Collagen in Diabetic Rat
- A comparison of anti-inflammatory activities of green tea and grapefruit seed extract with those of microencapsulated extracts
- Antimicrobial effects of green tea extract-containing dentifrice
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts