J Korean Soc Hypertens.
2013 Dec;19(4):123-131. 10.5646/jksh.2013.19.4.123.
Effects of Tamoxifen in Deoxycorticosterone Acetate-salt Hypertensive Nephropahty
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. skimw@chonnam.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 1838116
- DOI: http://doi.org/10.5646/jksh.2013.19.4.123
Abstract
- BACKGROUND
The present study was designed to evaluate the possible renoprotective effects of tamoxifen in deoxycorticosterone acetate (DOCA)-salt hypertensive (DSH) rats and its role in inflammation and fibrosis in the kidney.
METHODS
Male Sprague-Dawley rats, weighing 180 to 200 g, were used. All rats underwent unilateral nephrectomy. One week later, one group of rats (n = 8) was implanted with DOCA strips (200 mg/kg) and another group of rats (n = 8) was implanted with DOCA strips with co-treated with tamoxifen (10 mg/kg) through gavage feeding. Rats that did not implanted DOCA strips served as controls (n = 6). Two weeks later, the systolic blood pressure (SBP) was measured by tail-cuff method. The protein expression of transforming growth factor-beta (TGF-beta), Smad, alpha-smooth muscle actin (alpha-SMA), E-cadherin, ED-1, cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS) was determined in the kidney by immunoblotting. The mRNA expression of tumor necrosis factor-alpha (TNF-alpha), monocyte chemotactic protein-1 (MCP-1), and vascular cell adhesion molecule-1 (VCAM-1) was determined by real-time polymerase chain reaction.
RESULTS
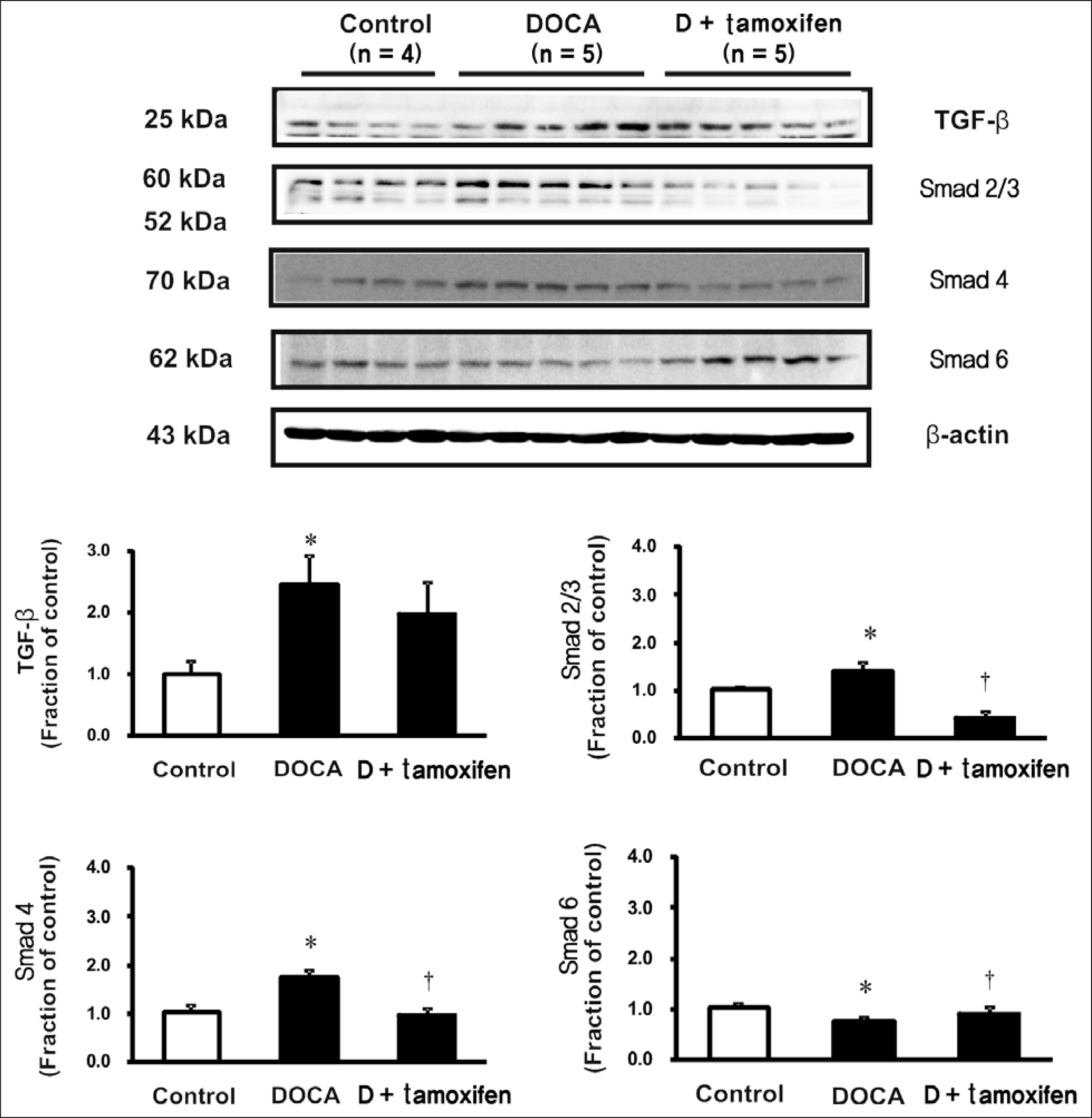
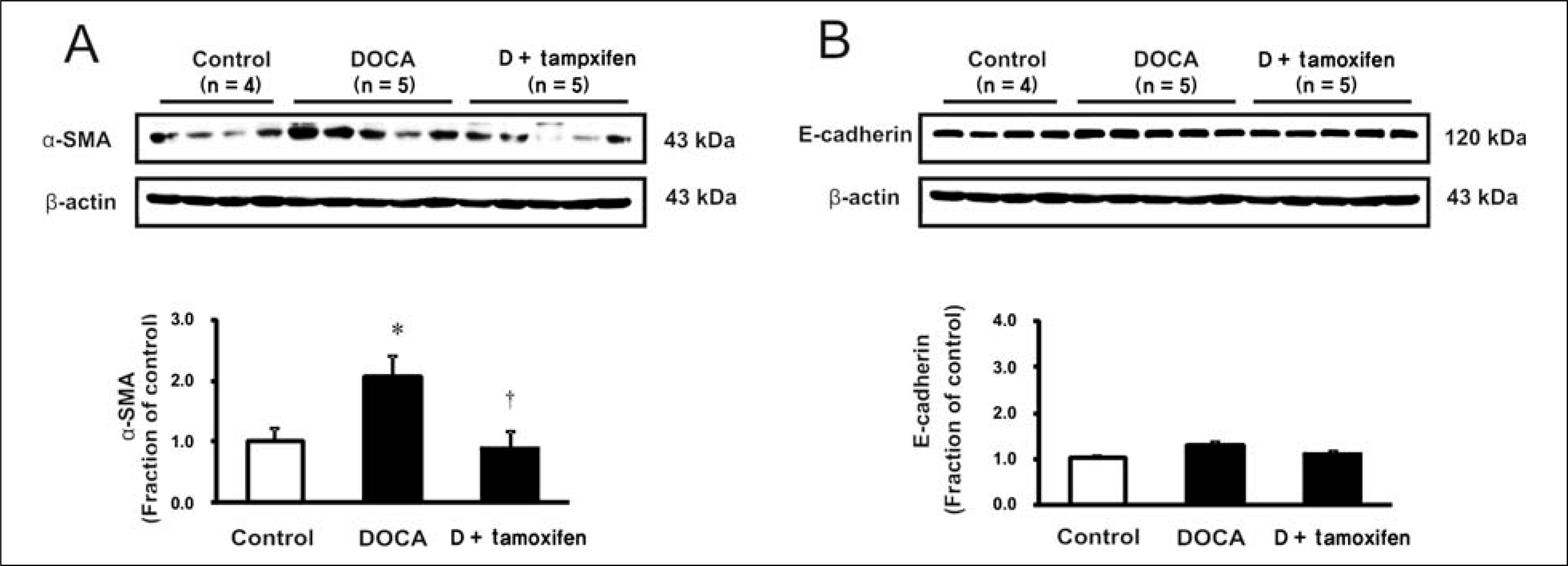
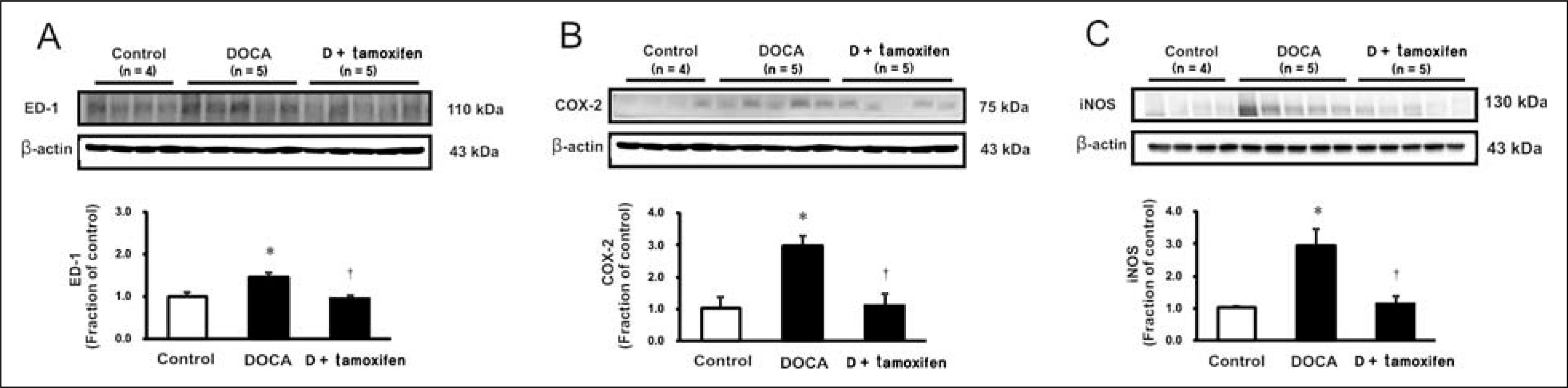
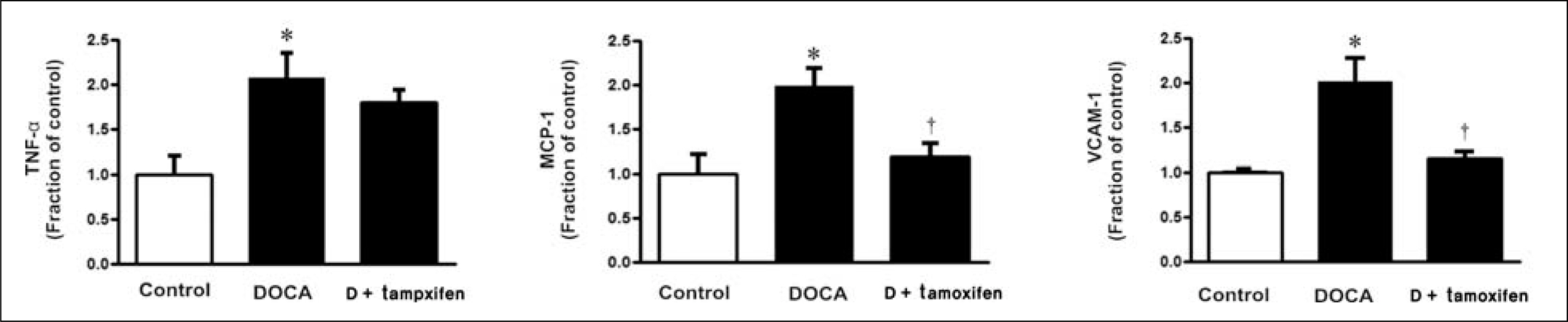
In DSH rats, SBP was increased, which was not affected by tamoxifen treatment. Serum creatinine level was comparable in DSH rats compared with controls, which was not affected by tamoxifen treatment. In DSH rats, the protein expression of TGF-beta, Smad 2/3, Smad 4, alpha-SMA, ED-1, COX-2, iNOS was increased compared with controls, and these changes were attenuated by tamoxifen treatment except that of TGF-beta. The mRNA expression of TNF-alpha, MCP-1, and VCAM-1 was increased, and expression of MCP-1 and VCAM-1 was counteracted by tamoxifen treatment.
CONCLUSIONS
Tamoxifen is effective in preventing the progression of nephropathy in DSH rats, the mechanism of which is associated with anti-inflammation and anti-fibrotic effects.
MeSH Terms
-
Actins
Animals
Blood Pressure
Cadherins
Chemokine CCL2
Creatinine
Cyclooxygenase 2
Desoxycorticosterone Acetate
Desoxycorticosterone*
Fibrosis
Humans
Hypertension
Immunoblotting
Inflammation
Kidney
Male
Methods
Muscles
Nephrectomy
Nitric Oxide Synthase Type II
Rats
Rats, Sprague-Dawley
Real-Time Polymerase Chain Reaction
RNA, Messenger
Tamoxifen*
Transforming Growth Factor beta
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Actins
Cadherins
Chemokine CCL2
Creatinine
Cyclooxygenase 2
Desoxycorticosterone
Nitric Oxide Synthase Type II
RNA, Messenger
Tamoxifen
Transforming Growth Factor beta
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Figure
Reference
-
References
1. Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006; 17:2275–84.
Article2. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007; 59:251–87.
Article3. Bae EH, Kim IJ, Ma SK, Kim SW. Rosiglitazone prevents the progression of renal injury in DOCA-salt hypertensive rats. Hypertens Res. 2010; 33:255–62.
Article4. Bae EH, Kim IJ, Park JW, Ma SK, Lee JU, Kim SW. Renoprotective effect of rosuvastatin in DOCA-salt hypertensive rats. Nephrol Dial Transplant. 2010; 25:1051–9.
Article5. Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med. 2004; 43:9–17.
Article6. Gavras H, Brunner HR, Laragh JH. Vaughan ED Jr, Koss M, Cote LJ, et al. Malignant hypertension resulting from deoxycorticosterone acetate and salt excess: role of renin and sodium in vascular changes. Circ Res. 1975; 36:300–9.7. Iglarz M, Touyz RM, Viel EC, Amiri F, Schiffrin EL. Involvement of oxidative stress in the profibrotic action of aldosterone: interaction wtih the renin-angiotension system. Am J Hypertens. 2004; 17:597–603.8. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002; 161:1773–81.9. Clark CP, Vanderpool D, Preskitt JT. The response of retroperitoneal fibrosis to tamoxifen. Surgery. 1991; 109:502–6.10. Korte MR, Yo M, Betjes MG, Fieren MW. van Saase JC, Boer WH, et al. Increasing incidence of severe encapsulating peritoneal sclerosis after kidney transplantation. Nephrol Dial Transplant. 2007; 22:2412–4.11. Van Bommel EF, Hendriksz TR, Huiskes AW, Zeegers AG. Brief communication: tamoxifen therapy for nonmalignant retroperitoneal fibrosis. Ann Intern Med. 2006; 144:101–6.
Article12. Neugarten J, Acharya A, Lei J, Silbiger S. Selective estrogen receptor modulators suppress mesangial cell collagen synthesis. Am J Physiol Renal Physiol. 2000; 279:F309–18.
Article13. Ruffy MB, Kunnavatana SS, Koch RJ. Effects of tamoxifen on normal human dermal fibroblasts. Arch Facial Plast Surg. 2006; 8:329–32.
Article14. Hu D, Hughes MA, Cherry GW. Topical tamoxifen: a potential therapeutic regime in treating excessive dermal scarring? Br J Plast Surg. 1998; 51:462–9.15. Delle H, Rocha JR, Cavaglieri RC, Vieira JM Jr, Malheiros DM, Noronha IL. Antifibrotic effect of tamoxifen in a model of progressive renal disease. J Am Soc Nephrol. 2012; 23:37–48.16. Bae EH, Kim IJ, Joo SY, Kim EY, Choi JS, Kim CS, et al. Renoprotective effects of the direct renin inhibitor aliskiren on gentamicin-induced nephrotoxicity in rats. J Renin Angiotensin Aldosterone Syst. 2013; Feb18 [Epub].
Article17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–8.18. Vielhauer V, Berning E, Eis V, Kretzler M, Segerer S, Strutz F, et al. CCR1 blockade reduces interstitial inflammation and fibrosis in mice with glomerulosclerosis and nephrotic syndrome. Kidney Int. 2004; 66:2264–78.
Article19. Liu Y, Tsuchihashi T, Kagiyama S, Matsumura K, Abe I, Fujishima M. Central and peripheral mechanisms involved in hypertension induced by chronic inhibition of nitric oxide synthase in rats. J Hypertens. 1998; 16:1165–73.20. Sigmon DH, Newman JM, Beierwaltes WH, Angiotensin II. endothelium-derived nitric oxide interaction in conscious rats. J Am Soc Nephrol. 1994; 4:1675–82.21. Murphy M, Crean J, Brazil DP, Sadlier D, Martin F, Godson C. Regulation and consequences of differential gene ex-pression in diabetic kidney disease. Biochem Soc Trans. 2008; 36(Pt 5):941–5.
Article22. Liu Z, Huang XR, Lan HY. Smad3 mediates ANG II-induced hypertensive kidney disease in mice. Am J Physiol Renal Physiol. 2012; 302:F986–97.
Article23. Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, et al. Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney Int. 2010; 77:1076–85.
Article24. Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, et al. Renoprotective effects of paricalcitol on gentamicin-induced kidney injury in rats. Am J Physiol Renal Physiol. 2010; 298:F301–13.
Article25. Allaria PM, Giangrande A, Gandini E, Pisoni IB. Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: tamoxifen as a new therapeutic agent? J Nephrol. 1999; 12:395–7.26. Balasubramaniam G, Brown EA, Davenport A, Cairns H, Cooper B, Fan SL, et al. The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol Dial Transplant. 2009; 24:3209–15.
Article27. Ergun I, Keven K, Canbakan B, Ekmekci Y, Erbay B. Tamoxifen in the treatment of idiopathic retroperitoneal fibrosis. Int Urol Nephrol. 2005; 37:341–3.
Article28. Nishi Y, Satoh M, Nagasu H, Kadoya H, Ihoriya C, Kidokoro K, et al. Selective estrogen receptor modulation attenuates proteinuria-induced renal tubular damage by modulating mitochondrial oxidative status. Kidney Int. 2013; 83:662–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of tyrosine kinases in vascular contraction in deoxycorticosterone acetate-salt hypertensive rats
- Tissue-specific regulation of angiotensinogen and angiotensin II receptor gene expression in deoxycorticosterone acetate-salt hypertensive rats
- Effects of Centrally Administered Losartan on Deoxycorticosterone-salt Hypertension Rats
- Coordinate expression of renin and cyclooxygenase-2 in rats with two-kidney, one clip and deoxycorticosterone acetate-salt hypertension
- Altered renal nitric oxide system in experimental hypertensive rats