Yonsei Med J.
2014 Sep;55(5):1333-1340. 10.3349/ymj.2014.55.5.1333.
Histone Acetylation Level and Histone Acetyltransferase/Deacetylase Activity in Ejaculated Sperm from Normozoospermic Men
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea. blasto@snubh.org
- 2Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Seoul National University Hospital, Seoul, Korea.
- KMID: 1799499
- DOI: http://doi.org/10.3349/ymj.2014.55.5.1333
Abstract
- PURPOSE
The aim of this work was to evaluate nuclear histone acetylation level and total histone acetyltransferase (HAT) and deacetylase (HDAC) activity in ejaculated sperm and their relevance to conventional sperm parameters.
MATERIALS AND METHODS
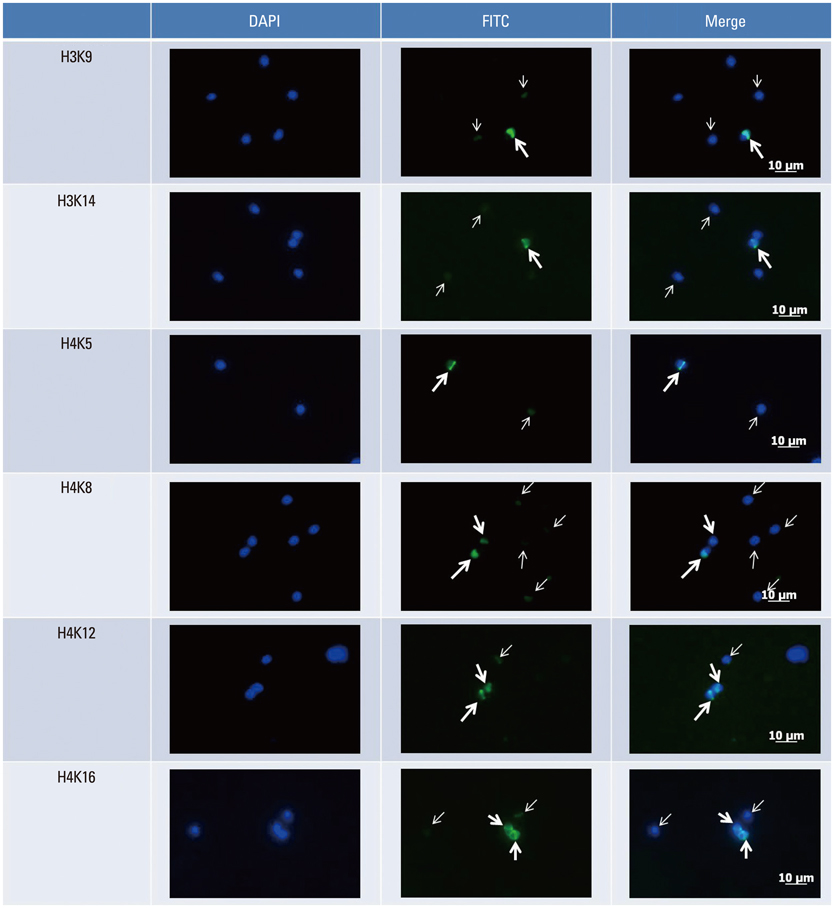
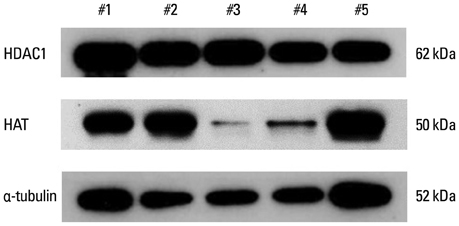
Thirty-three normozoospermic men were included in this study. Semen samples were processed by swim-up and then immunostained by six acetylation antibodies (H3K9ac, H3K14ac, H4K5ac, H4K8ac, H4K12ac, and H4K16ac). Our preliminary study verified the expression of HAT/HDAC1 in mature human sperm. From vitrified-warmed sperm samples, total HAT/HDAC activity was measured by commercially available kits. Nuclear DNA integrity was also measured by TUNEL assay.
RESULTS
The levels of six acetylation marks were not related with conventional sperm parameters including sperm DNA fragmentation index (DFI) as well as HAT/HDAC activity. However, sperm DFI was positively correlated with HAT activity (r=0.038 after adjustment, p<0.02). HAT activity showed a negative relationship with HDAC activity (r=-0.51, p<0.01). Strict morphology was negatively correlated with acetylation enzyme index (=HAT activity/HDAC activity) (r=-0.53, p<0.01).
CONCLUSION
Our works demonstrated a significant relationship of acetylation-associated enzyme activity and strict morphology or sperm DFI.
Keyword
MeSH Terms
Figure
Reference
-
1. Akiyama T, Nagata M, Aoki F. Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. Proc Natl Acad Sci U S A. 2006; 103:7339–7344.
Article2. Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006; 31:89–97.
Article3. Ma P, Schultz RM. Histone deacetylase 1 (HDAC1) regulates histone acetylation, development, and gene expression in preimplantation mouse embryos. Dev Biol. 2008; 319:110–120.
Article4. Omisanjo OA, Biermann K, Hartmann S, Heukamp LC, Sonnack V, Hild A, et al. DNMT1 and HDAC1 gene expression in impaired spermatogenesis and testicular cancer. Histochem Cell Biol. 2007; 127:175–181.
Article5. Davie JR. Covalent modifications of histones: expression from chromatin templates. Curr Opin Genet Dev. 1998; 8:173–178.
Article6. Turner BM. Histone acetylation and control of gene expression. J Cell Sci. 1991; 99(Pt 1):13–20.
Article7. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003; 370(Pt 3):737–749.
Article8. Spencer VA, Davie JR. Role of covalent modifications of histones in regulating gene expression. Gene. 1999; 240:1–12.
Article9. Fenic I, Sonnack V, Failing K, Bergmann M, Steger K. In vivo effects of histone-deacetylase inhibitor trichostatin-A on murine spermatogenesis. J Androl. 2004; 25:811–818.
Article10. Hazzouri M, Pivot-Pajot C, Faure AK, Usson Y, Pelletier R, Sèle B, et al. Regulated hyperacetylation of core histones during mouse spermatogenesis: involvement of histone deacetylases. Eur J Cell Biol. 2000; 79:950–960.
Article11. Kistler WS, Henriksén K, Mali P, Parvinen M. Sequential expression of nucleoproteins during rat spermiogenesis. Exp Cell Res. 1996; 225:374–381.
Article12. Sonnack V, Failing K, Bergmann M, Steger K. Expression of hyperacetylated histone H4 during normal and impaired human spermatogenesis. Andrologia. 2002; 34:384–390.
Article13. Faure AK, Pivot-Pajot C, Kerjean A, Hazzouri M, Pelletier R, Péoc'h M, et al. Misregulation of histone acetylation in Sertoli cell-only syndrome and testicular cancer. Mol Hum Reprod. 2003; 9:757–763.
Article14. Gatewood JM, Cook GR, Balhorn R, Schmid CW, Bradbury EM. Isolation of four core histones from human sperm chromatin representing a minor subset of somatic histones. J Biol Chem. 1990; 265:20662–20666.
Article15. Steger K, Cavalcanti MC, Schuppe HC. Prognostic markers for competent human spermatozoa: fertilizing capacity and contribution to the embryo. Int J Androl. 2011; 34(6 Pt 1):513–527.
Article16. Dada R, Kumar M, Jesudasan R, Fernández JL, Gosálvez J, Agarwal A. Epigenetics and its role in male infertility. J Assist Reprod Genet. 2012; 29:213–223.
Article17. Emery BR, Carrell DT. The effect of epigenetic sperm abnormalities on early embryogenesis. Asian J Androl. 2006; 8:131–142.
Article18. Grunewald S, Paasch U, Glander HJ, Anderegg U. Mature human spermatozoa do not transcribe novel RNA. Andrologia. 2005; 37:69–71.
Article19. Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009; 460:473–478.
Article20. Krawetz SA. Paternal contribution: new insights and future challenges. Nat Rev Genet. 2005; 6:633–642.
Article21. Sutovsky P, Schatten G. Paternal contributions to the mammalian zygote: fertilization after sperm-egg fusion. Int Rev Cytol. 2000; 195:1–65.
Article22. Hecht N, Behr R, Hild A, Bergmann M, Weidner W, Steger K. The common marmoset (Callithrix jacchus) as a model for histone and protamine expression during human spermatogenesis. Hum Reprod. 2009; 24:536–545.
Article23. Rousseaux S, Reynoird N, Escoffier E, Thevenon J, Caron C, Khochbin S. Epigenetic reprogramming of the male genome during gametogenesis and in the zygote. Reprod Biomed Online. 2008; 16:492–503.
Article24. Steilmann C, Paradowska A, Bartkuhn M, Vieweg M, Schuppe HC, Bergmann M, et al. Presence of histone H3 acetylated at lysine 9 in male germ cells and its distribution pattern in the genome of human spermatozoa. Reprod Fertil Dev. 2011; 23:997–1011.
Article25. Jee BC, Suh CS, Shin MS, Lee HJ, Lee JH, Kim SH. Sperm nuclear DNA fragmentation and chromatin structure in one-day-old ejaculated sperm. Clin Exp Reprod Med. 2011; 38:82–86.
Article26. Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004; 19:611–615.
Article27. Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012; 27:2908–2917.
Article28. Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010; 93:1027–1036.
Article29. Grozinger CM, Schreiber SL. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem Biol. 2002; 9:3–16.30. Delaval K, Govin J, Cerqueira F, Rousseaux S, Khochbin S, Feil R. Differential histone modifications mark mouse imprinting control regions during spermatogenesis. EMBO J. 2007; 26:720–729.
Article31. Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012; 98:1400–1406.32. Künzle R, Mueller MD, Hänggi W, Birkhäuser MH, Drescher H, Bersinger NA. Semen quality of male smokers and nonsmokers in infertile couples. Fertil Steril. 2003; 79:287–291.
Article33. Pasqualotto FF, Umezu FM, Salvador M, Borges E Jr, Sobreiro BP, Pasqualotto EB. Effect of cigarette smoking on antioxidant levels and presence of leukocytospermia in infertile men: a prospective study. Fertil Steril. 2008; 90:278–283.
Article34. Said TM, Ranga G, Agarwal A. Relationship between semen quality and tobacco chewing in men undergoing infertility evaluation. Fertil Steril. 2005; 84:649–653.
Article35. Carrell DT. Contributions of spermatozoa to embryogenesis: assays to evaluate their genetic and epigenetic fitness. Reprod Biomed Online. 2008; 16:474–484.
Article36. Tesarik J. Paternal effects on cell division in the human preimplantation embryo. Reprod Biomed Online. 2005; 10:370–375.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Histone Acetylation in Mesenchymal Stem Cell Differentiation
- HDAC and HDAC Inhibitor: From Cancer to Cardiovascular Diseases
- Activation of ATM-dependent DNA Damage Signal Pathway by a Histone Deacetylase Inhibitor, Trichostatin A
- Expression of HAT1 and HDAC1, 2, 3 in Diffuse Large B-Cell Lymphomas, Peripheral T-Cell Lymphomas, and NK/T-Cell Lymphomas
- A Novel Osteogenic Activity of Suberoylanilide Hydroxamic Acid is Synergized by BMP-2