Korean J Physiol Pharmacol.
2015 May;19(3):257-262. 10.4196/kjpp.2015.19.3.257.
Nicotine in High Concentration Causes Contraction of Isolated Strips of Rabbit Corpus Cavernosum
- Affiliations
-
- 1Advanced Urogenital Disease Research Center; Research Institute for Translational System Biomics; Department of Urology, Chung-Ang University Hospital, Seoul 156-755, Korea. uromyung@cau.ac.kr
- 2Department of Urology, Seoul Medical Center, Seoul 131-795, Korea.
- 3Department of Physiology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea. heeyun@cau.ac.kr
- 4Department of Urology, Hallym University Dongtan Sacred Heart Hospital, Hwaseong 445-170, Korea.
- KMID: 1791427
- DOI: http://doi.org/10.4196/kjpp.2015.19.3.257
Abstract
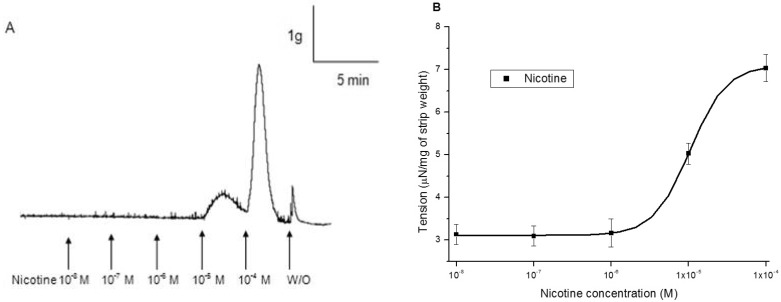
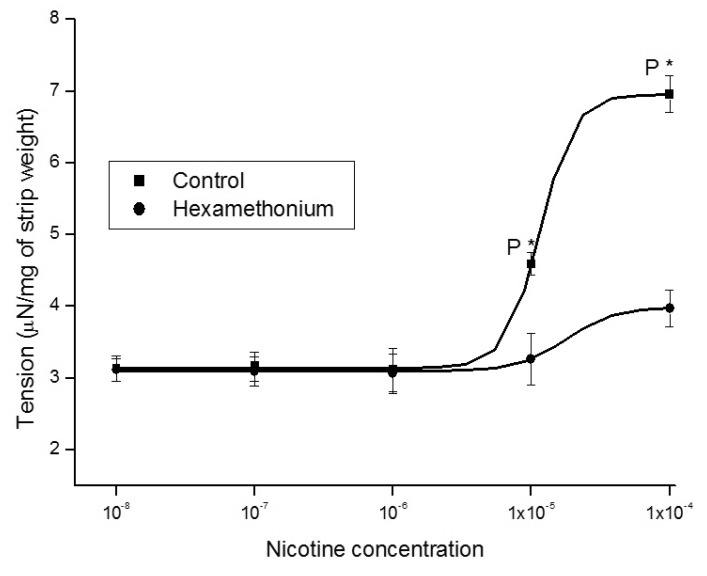
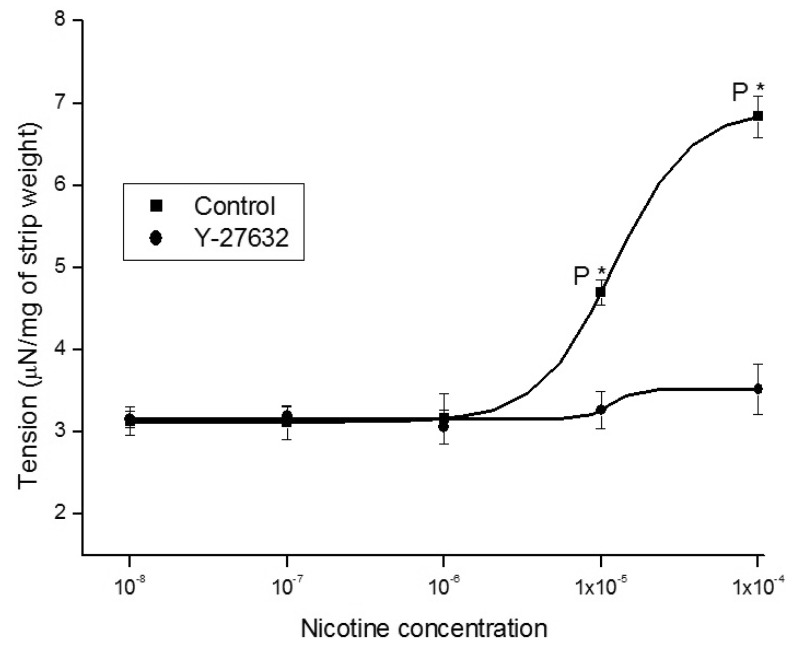
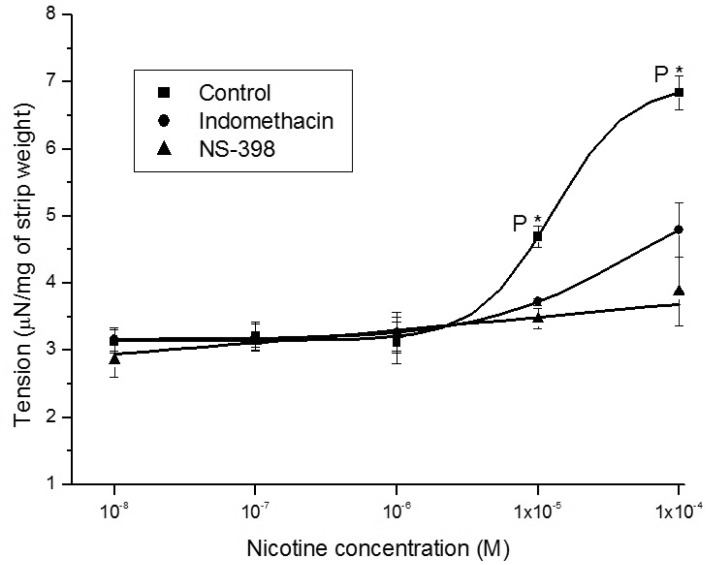
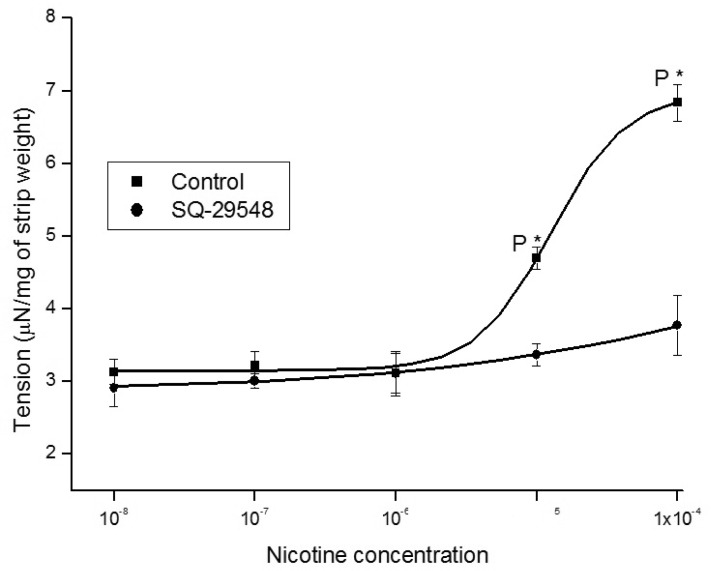
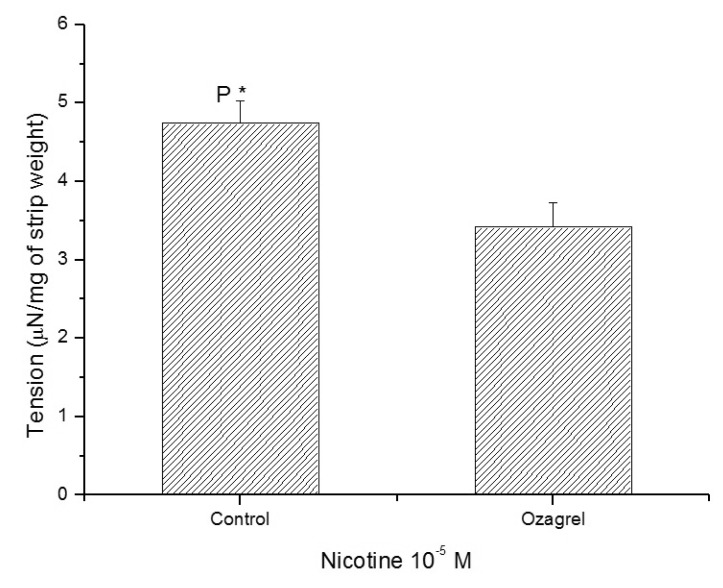
- It is well known that cigarette smoke can cause erectile dysfunction by affecting the penile vascular system. However, the exact effects of nicotine on the corpus cavernosum remains poorly understood. Nicotine has been reported to cause relaxation of the corpus cavernosum; it has also been reported to cause both contraction and relaxation. Therefore, high concentrations of nicotine were studied in strips from the rabbit corpus cavernosum to better understand its effects. The proximal penile corpus cavernosal strips from male rabbits weighing approximately 4 kg were used in organ bath studies. Nicotine in high concentrations (10(-5)~10(-4) M) produced dose-dependent contractions of the corpus cavernosal strips. The incubation with 10(-5) M hexamethonium (nicotinic receptor antagonist) significantly inhibited the magnitude of the nicotine associated contractions. The nicotine-induced contractions were not only significantly inhibited by pretreatment with 10(-5) M indomethacin (nonspecific cyclooxygenase inhibitor) and with 10(-6) M NS-398 (selective cyclooxygenase inhibitor), but also with 10(-6) M Y-27632 (Rho kinase inhibitor). Ozagrel (thromboxane A2 synthase inhibitor) and SQ-29548 (highly selective TP receptor antagonist) pretreatments significantly reduced the nicotine-induced contractile amplitude of the strips. High concentrations of nicotine caused contraction of isolated rabbit corpus cavernosal strips. This contraction appeared to be mediated by activation of nicotinic receptors. Rho-kinase and cyclooxygenase pathways, especially cyclooxygenase-2 and thromboxane A2, might play a pivotal role in the mechanism associated with nicotine-induced contraction of the rabbit corpus cavernosum.
MeSH Terms
-
Baths
Cyclooxygenase 2
Erectile Dysfunction
Hexamethonium
Humans
Indomethacin
Male
Nicotine*
Phosphotransferases
Prostaglandin-Endoperoxide Synthases
Rabbits
Receptors, Nicotinic
Receptors, Thromboxane
Relaxation
rho-Associated Kinases
Smoke
Thromboxane A2
Tobacco Products
Cyclooxygenase 2
Hexamethonium
Indomethacin
Nicotine
Phosphotransferases
Prostaglandin-Endoperoxide Synthases
Receptors, Nicotinic
Receptors, Thromboxane
Smoke
Thromboxane A2
rho-Associated Kinases
Figure
Reference
-
1. McVary KT, Carrier S, Wessells H. Subcommittee on Smoking and Erectile Dysfunction Socioeconomic Committee. Sexual Medicine Society of North America. Smoking and erectile dysfunction: evidence based analysis. J Urol. 2001; 166:1624–1632. PMID: 11586190.
Article2. Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989; 320:1025–1030. PMID: 2927481.
Article3. Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J Clin. 2005; 55:281–299. PMID: 16166074.
Article4. Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005; 57:79–115. PMID: 15734728.
Article5. Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996; 36:597–613. PMID: 8725403.
Article6. Zainalabidin S, Budin SB, Ramalingam A, Lim YC. Aortic remodelling in chronic nicotine-administered rat. Korean J Physiol Pharmacol. 2014; 18:411–418. PMID: 25352761.
Article7. Pickering TG, Schwartz JE, James GD. Ambulatory blood pressure monitoring for evaluating the relationships between lifestyle, hypertension and cardiovascular risk. Clin Exp Pharmacol Physiol. 1995; 22:226–231. PMID: 7554420.
Article8. Gunby P. Surgeon General emphasizes nicotine addiction in annual report on tobacco use, consequences. JAMA. 1988; 259:2811. PMID: 3367442.
Article9. Hanna ST. Nicotine effect on cardiovascular system and ion channels. J Cardiovasc Pharmacol. 2006; 47:348–358. PMID: 16633075.10. Hayashida H, Okamura T, Tomoyoshi T, Toda N. Neurogenic nitric oxide mediates relaxation of canine corpus cavernosum. J Urol. 1996; 155:1122–1127. PMID: 8583577.
Article11. Bagcivan I, Gokce G, Yildirim S, Sarioglu Y. Investigation of the mechanism of nicotine induced relaxation in rabbit corpus cavernosum in vitro. Urol Res. 2004; 32:209–212. PMID: 15205855.
Article12. Klinge E, Alaranta S, Sjöstrand NO. Pharmacological analysis of nicotinic relaxation of bovine retractor penis muscle. J Pharmacol Exp Ther. 1988; 245:280–286. PMID: 3361446.13. Bozkurt NB, Vural IM, Sarioglu Y, Pekiner C. Nicotine potentiates the nitrergic relaxation responses of rabbit corpus cavernosum tissue via nicotinic acetylcholine receptors. Eur J Pharmacol. 2007; 558:172–178. PMID: 17208220.
Article14. Hayashida H, Fujimoto H, Yoshida K, Tomoyoshi T, Okamura T, Toda N. Comparison of neurogenic contraction and relaxation in canine corpus cavernosum and penile artery and vein. Jpn J Pharmacol. 1996; 72:231–240. PMID: 8957684.
Article15. Ozturk Fincan GS, Vural IM, Ercan ZS, Sarioglu Y. Enhancement effects of nicotine on neurogenic relaxation responses in the corpus cavernosum in rabbits: the role of nicotinic acetylcholine receptor subtypes. Eur J Pharmacol. 2010; 627:281–284. PMID: 19878666.
Article16. Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009; 89:73–120. PMID: 19126755.
Article17. Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003; 27:201–206. PMID: 14627618.
Article18. Rees RW, Ralph DJ, Royle M, Moncada S, Cellek S. Y-27632, an inhibitor of Rho-kinase, antagonizes noradrenergic contractions in the rabbit and human penile corpus cavernosum. Br J Pharmacol. 2001; 133:455–458. PMID: 11399661.
Article19. Wingard CJ, Husain S, Williams J, James S. RhoA-Rho kinase mediates synergistic ET-1 and phenylephrine contraction of rat corpus cavernosum. Am J Physiol Regul Integr Comp Physiol. 2003; 285:R1145–R1152. PMID: 12893655.
Article20. Needleman P, Moncada S, Bunting S, Vane JR, Hamberg M, Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature. 1976; 261:558–560. PMID: 934294.
Article21. Clària J. Cyclooxygenase-2 biology. Curr Pharm Des. 2003; 9:2177–2190. PMID: 14529398.22. Crofford LJ, Lipsky PE, Brooks P, Abramson SB, Simon LS, van de. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum. 2000; 43:4–13. PMID: 10643694.
Article23. Ji X, Nishihashi T, Trandafir CC, Wang A, Shimizu Y, Kurahashi K. Pharmacological nature of nicotine-induced contraction in the rat basilar artery: involvement of arachidonic acid metabolites. Eur J Pharmacol. 2007; 577:109–114. PMID: 17765890.
Article24. Daley JT, Brown ML, Watkins T, Traish AM, Huang YH, Moreland RB, De Tejada IS. Prostanoid production in rabbit corpus cavernosum: I. regulation by oxygen tension. J Urol. 1996; 155:1482–1487. PMID: 8632615.
Article25. Daley JT, Watkins MT, Brown ML, Martinez V, Cuevas P, Saenz de. Prostanoid production in rabbit corpus cavernosum. II. Inhibition by oxidative stress. J Urol. 1996; 156:1169–1173. PMID: 8709340.26. Moreland RB, Albadawi H, Bratton C, Patton G, Goldstein I, Traish A, Watkins MT. O2-dependent prostanoid synthesis activates functional PGE receptors on corpus cavernosum smooth muscle. Am J Physiol Heart Circ Physiol. 2001; 281:H552–H558. PMID: 11454556.
Article27. Angulo J, Cuevas P, La Fuente JM, Pomerol JM, Ruiz-Castañé E, Puigvert A, Gabancho S, Fernández A, Ney P, Sáenz De. Regulation of human penile smooth muscle tone by prostanoid receptors. Br J Pharmacol. 2002; 136:23–30. PMID: 11976264.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Endothelium Dependent Vasodilator Substances on Rabbit Corpora Cavernosa
- Pharmacologic Effect of Phentolamine on Norepinephrine Induced Contraction of Corpus Cavernosum
- Effect of Glucocorticoid on Rabbit Corpus Cavernosum Smooth Muscle
- Effects of Angiotensin III in Rabbit Corpus Cavernosum Smooth Muscle Contraction: Comparing with Angiotensin I and Angiotensin II
- Effects of Ginseng Alkaloid on the Tension of Rabbit Corpus Cavernosum