J Korean Med Sci.
2008 Oct;23(5):870-876. 10.3346/jkms.2008.23.5.870.
Overexpression of USF Increases TGF-beta1 Protein Levels, But G1 Phase Arrest was not Induced in FRTL-5 Cells
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jhchung@smc.samsung.co.kr
- 2Samsung Biomedical Research Institute, Seoul, Korea.
- 3Division of Endocrinology, Department of Internal Medicine, College of Medicine, Chung-Ang University, Seoul, Korea.
- KMID: 1783075
- DOI: http://doi.org/10.3346/jkms.2008.23.5.870
Abstract
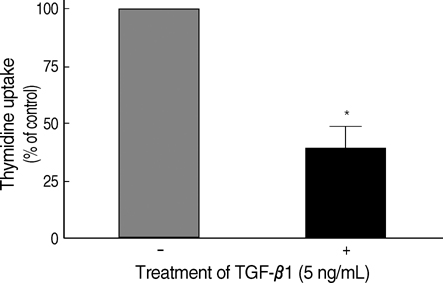
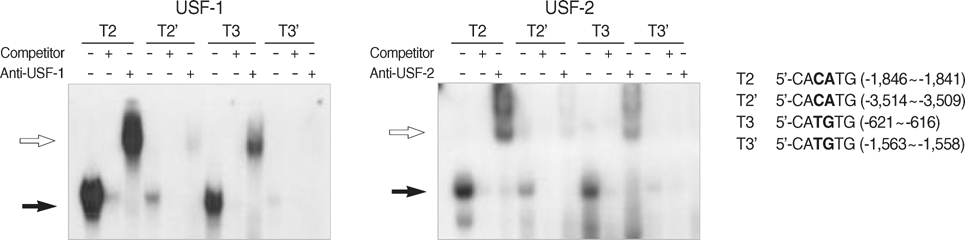
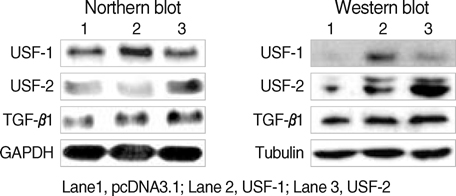
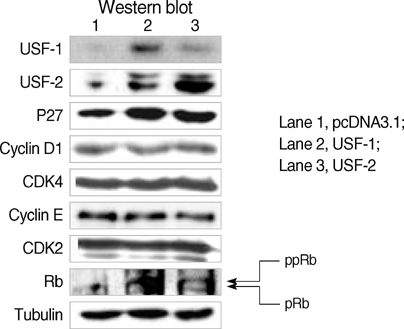
- Transforming growth factor-beta1 (TGF-beta1) is a potent inhibitor of cellular growth and proliferation by G1 phase arrest or apoptosis. We investigated the association of TGF-beta1 with the anti-proliferative effect of upstream stimulatory factor (USF) in Fischer rat thyroid cell line (FRTL-5) cells. [Methyl-(3)H] thymidine uptake was measured after treatment of FRTL-5 cells with TGF-beta1 to identify its anti-proliferative effect. USF-1 and USF-2 proteins were in vitro translated, and an electrophoretic mobility shift assay was performed to identify the interaction between USF and the TGF-beta1 promoter. FRTL-5 cells were transfected with USF cDNA, and then the expression of TGF-beta1 was examined with Northern and Western blotting. The cell cycle-regulating proteins associated with TGF-beta1 were also measured. TGF-beta1 significantly inhibited [methyl-(3)H] thymidine uptake in FRTL-5 cells. Two specific binding sites for USF were found in the TGF-beta1 promoter: -1,846~-1,841 (CACATG) and -621~-616 (CATGTG). Overexpression of USF increased both the mRNA levels and protein levels of TGF-beta1. However, the expression of cyclin D1, CDK4, cyclin E, and CDK2, and the phosphorylation of retinoblastoma protein remained unchanged. Overexpression of USF in FRTL-5 cells increased the expression of TGF-beta10 through specific binding to TGF-beta1 promoter. However, the USF-induced expression of TGF-beta1 did not cause G1 arrest.
MeSH Terms
Figure
Reference
-
1. Sawadogo M, Roeder RG. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985. 43:165–175.
Article2. Carthew RW, Chodosh LA, Sharp PA. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985. 43:439–448.
Article3. Sawadogo M, Van Dyke MW, Gregor PD, Roeder RG. Multiple forms of the human gene-specific transcription factor USF. I. Complete purification and identification of USF from HeLa cell nuclei. J Biol Chem. 1988. 263:11985–11993.
Article4. Sawadogo M. Multiple forms of the human gene-specific transcription factor USF. II. DNA binding properties and transcriptional activity of the purified HeLa USF. J Biol Chem. 1988. 263:11994–12001.
Article5. Gregor PD, Sawadogo M, Roeder RG. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimmer. Genes Dev. 1990. 4:1730–1740.6. Sirito M, Walker S, Lin Q, Kozlowski MT, Klein WH, Sawadogo M. Members of the USF family of helix-loop-helix proteins bind DNA as homo- as well as heterodimers. Gene Expr. 1992. 2:231–240.7. Miyamoto NG, Moncollin V, Wintzerith M, Hen R, Egly JM, Chambon P. Stimulation of in vitro transcription by the upstream element of the adenovirus-2 major late promoter involves a specific factor. Nucleic Acids Res. 1984. 12:8779–8799.8. Yu YT, Manley JL. Generation and functional analyses for base-substitution mutants of the adenovirus 2 major late promoter. Nucleic Acids Res. 1984. 12:9309–9321.
Article9. Luo X, Sawadogo M. Antiproliferative properties of the USF family of helix-loop-helix transcription factors. Proc Natl Acad Sci USA. 1996. 93:1308–1313.
Article10. Qyang Y, Luo X, Lu T, Ismail PM, Krylov D, Vinson C, Sawadogo M. Cell-type-dependent activity of the ubiquitous transcription factor USF in cellular proliferation and transcriptional activation. Mol Cell Biol. 1999. 19:1508–1517.
Article11. Jung HS, Kim KS, Chung YJ, Chung HK, Min YK, Lee MS, Lee MK, Kim KW, Chung JH. USF inhibits cell proliferation through delay in G2/M phase in FRTL-5 cells. Endocr J. 2007. 54:275–285.
Article12. Glick AB. TGF beta1 back to the future: revisiting its role as a transforming growth factor. Cancer Biol Ther. 2004. 3:276–283.13. Kaklamani VG, Pasche B. Role of TGF-beta in cancer and the potential for therapy and prevention. Expert Rev Anticancer Ther. 2004. 4:649–661.14. Sun L. Tumor-suppressive and promoting function of transforming growth factor beta. Front Biosci. 2004. 9:1925–1935.
Article15. Pang XP, Park M, Hershman JM. Transforming growth factor-beta blocks protein kinase-A-mediated iodide transport and protein kinase-C-mediated DNA synthesis in FRTL-5 rat thyroid cells. Endocrinology. 1992. 131:45–50.
Article16. Coppa A, Mincione G, Mammarella S, Ranieri A, Colletta G. Epithelial rat thyroid cell clones, escaping from transforming growth factor beta negative growth control, are still inhibited by this factor in the ability to trap iodide. Cell Growth Differ. 1995. 6:281–290.17. Asmis LM, Kaempf J, Von Gruenigen C, Kimura ET, Wagner HE, Studer H. Acquired and naturally occurring resistance of thyroid follicular cells to the growth inhibitory action of transforming growth factor-beta 1 (TGF-beta 1). J Endocrinol. 1996. 149:485–496.18. Carneiro C, Alvarez CV, Zalvide J, Vidal A, Dominguez F. TGF-beta1 actions on FRTL-5 cells provide a model for the physiological regulation of thyroid growth. Oncogene. 1998. 16:1455–1465.19. Weigert C, Brodbeck K, Sawadogo M, Haring HU, Schleicher ED. Upstream stimulatory factor (USF) proteins induce human TGF-beta1 gene activation via the glucose-response element-1013/-1002 in mesangial cells: up-regulation of USF activity by the hexosamine biosynthetic pathway. J Biol Chem. 2004. 279:15908–15915.20. Pang XP, Hershman JM. Transforming growth factor-beta1 resistance in a thyroid cancer model of tumor necrosis factor-alpha resistance. Thyroid. 1998. 8:1065–1070.21. Scholtz B, Kingsley-Kallesen M, Rizzino A. Transcription of the transforming growth factor-beta2 gene is dependent on an E-box located between an essential cAMP response element/activating transcription factor motif and the TATA box of the gene. J Biol Chem. 1996. 271:32375–32380.22. Kingsley-Kallesen M, Luster TA, Rizzino A. Transcriptional regulation of the transforming growth factor-beta2 gene in glioblastoma cells. In Vitro Cell Dev Biol Anim. 2001. 37:684–690.23. Ravitz MJ, Wenner CE. Cyclin-dependent kinase regulation during G1 phase and cell cycle regulation by TGF-beta. Adv Cancer Res. 1997. 71:165–207.24. Massague J, Blain SW, Lo RS. TGF beta signaling in growth control, cancer, and heritable disorders. Cell. 2000. 103:295–309.25. Ewen ME, Sloss HK, Whitehouse LL, Livingston DM. TGF beta inhibition of CDK4 synthesis is linked to cell cycle arrest. Cell. 1993. 74:1009–1020.26. Iavarone A, Massague J. Repression of the CDK activator cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature. 1997. 387:417–422.27. Warner BJ, Blain SW, Seoane J, Massague J. Myc downregulation by transforming growth factor beta required for activation of the p15 (link4b) G(1) arrest pathway. Mol Cell Biol. 1999. 19:5913–5922.28. Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995. 81:323–330.
Article29. Zetterberg A, Larsson O, Wiman KG. What is the restriction point? Curr Opin Cell Biol. 1995. 7:835–842.
Article30. Hashimoto O, Ueno T, Kimura R, Ohtsubo M, Nakamura T, Koga H, Torimura T, Uchida S, Yamashita K, Sata M. Inhibition of proteasome-dependent degradation of Wee1 in G2-arrested Hep3B cells by TGF beta 1. Mol Carcinog. 2003. 36:171–182.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Experimental Study of the Effect of TGF-beta1 on the Growth and Apoptosis in Y-79 Retinoblastoma Cells
- TGF-beta1 induces mouse dendritic cells to express VEGF and its receptor (Flt-1) under hypoxic conditions
- The Inhibitory Effect of siRNAs on The High Glucose-Induced Overexpression of TGF-beta1 in Mesangial Cells
- Effects of USF-1, USF-2, PTEN and Thyroid Transcription Factors on the Function and Growth in FRTL-5 Cells
- Expression of Transforming Growth Factor-beta1 , beta2 by Immunohistochemical Staining method: In Human Endometrium through the Menstrual Cycle