Yonsei Med J.
2008 Dec;49(6):917-922. 10.3349/ymj.2008.49.6.917.
Decreased Expression of 15-hydroxyprostaglandin Dehydrogenase in Gastric Carcinomas
- Affiliations
-
- 1Department of Pathology, Dongguk University College of Medicine, Kyongju, Korea. taejung@mail.dongguk.ac.kr
- 2Department of General Surgery, Dongguk University College of Medicine, Kyongju, Korea.
- KMID: 1782939
- DOI: http://doi.org/10.3349/ymj.2008.49.6.917
Abstract
- PURPOSE
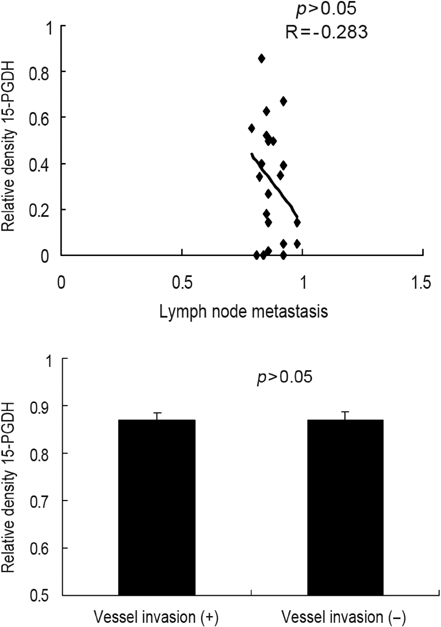
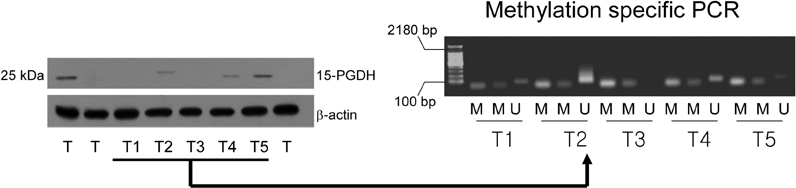
Gastric carcinoma tissues release high level of prostaglandin E2 (PGE2) when compared to non-neoplastic mucosa, and cyclooxygenase-2 (COX-2), which is the rate-limiting enzyme in prostaglandin (PG) biosynthesis, is often overexpressed in gastric carcinomas and during gastric carcinogenesis. However, little is known about the expression of 15-hydroxyprostaglandin dehydrogenase (15-PGDH), the key enzyme responsible for the biological inactivation of PG, in gastric carcinomas. MATERIALS AND METHODS: We investigated the expression of 15-PGDH in 28 cases of advanced gastric carcinomas by Western blot analysis and also the relation between its expression and the gene promoter methylation. RESULTS: 15-PGDH expression was significantly decreased in gastric carcinomas compared to corresponding non-neoplastic tissues and inversely correlated with the expression of proliferating cell nuclear antigen in gastric carcinomas. However, there was no correlation between 15-PGDH expression and pathological findings such as nodal metastasis and vascular invasion. Promoter hypermethylation of 15-PGDH gene was not detected in carcinomas, with only a negligible expression of the enzyme. CONCLUSION: Our results suggested that 15-PGDH has tumor suppressor activity in gastric carcinomas.
MeSH Terms
Figure
Reference
-
1. Eberhart CE, Dubois RN. Eicosanoids and gastrointestinal tract. Gastroenterology. 1995. 109:285–301.2. Uefuji K, Ichikura T, Mochizuki H. Cyclooxygenase-2 expression is related to prostaglandin biosynthesis and angiogenesis in human gastric cancer. Clin Cancer Res. 2000. 6:135–138.3. DeWitt DL. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta. 1991. 1083:121–134.
Article4. Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999. 5:1418–1423.
Article5. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995. 83:493–501.
Article6. Tsujii M, Kawano S, DuBois RN. Cyclooxyenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997. 94:3336–3340.
Article7. Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998. 93:705–716.
Article8. Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997. 57:1276–1280.9. Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, et al. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999. 94:451–455.10. Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, et al. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000. 6:519–525.11. Jang TJ. Expression of proteins related to prostaglandin E2 biosynthesis is increased in human gastric cancer and during gastric carcinogenesis. Virchows Arch. 2004. 445:564–571.
Article12. Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002. 68-69:483–493.
Article13. Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-bata-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004. 101:17468–17473.
Article14. Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005. 280:3217–3223.
Article15. Ding Y, Tong M, Liu S, Moscow JA, Tai HH. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005. 26:65–72.
Article16. Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006. 103:12098–12102.17. Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, et al. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006. 66:7818–7823.
Article18. Yoo NJ, Jeong EG, Lee SH, Lee SH. Expression of 15-hydroxyprostaglandin dehydrogenase, a COX-2 antagonist and tumour suppressor, is not altered in gastric carcinomas. Pathology. 2007. 39:174–175.
Article19. Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG island. Proc Natl Acad Sci U S A. 1996. 93:9821–9826.
Article20. Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res. 2005. 65:4218–4227.21. Yang L, Amann JM, Kikuchi T, Porta R, Guix M, Gonzalez A, et al. Inhibition of epidermal growth factor receptor signaling elevates 15-hydroxyprostaglandin dehydrogenase in non-small-cell lung cancer. Cancer Res. 2007. 67:5587–5593.
Article22. Mann JR, Backlund MG, Buchanan FG, Daikoku T, Holla VR, Rosenberg DW, et al. Repression of prostaglandin dehydrogenase by epidermal growth factor and snail increases prostaglandin E2 and promotes cancer progression. Cancer Res. 2006. 66:6649–6656.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between 15-hydroxyprostaglandin dehydrogenase and gastric adenocarcinoma
- Prognostic Implication of 15-Hydroxyprostaglandin Dehydrogenase Down-Regulation in Patients with Colorectal Cancer
- Role of 15-hydroxyprostaglandin dehydrogenase down-regulation on the prognosis of hepatocellular carcinoma
- 15-Hydroxyprostaglandin dehydrogenase as a marker in colon carcinogenesis: analysis of the prostaglandin pathway in human colonic tissue
- 15-Hydroxyprostaglandin Dehydrogenase in Colorectal Mucosa as a Potential Biomarker for Predicting Colorectal Neoplasms