Intest Res.
2017 Jan;15(1):75-82. 10.5217/ir.2017.15.1.75.
15-Hydroxyprostaglandin dehydrogenase as a marker in colon carcinogenesis: analysis of the prostaglandin pathway in human colonic tissue
- Affiliations
-
- 1Department of Gastroenteroloy, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sjmyung@amc.seoul.kr
- 2Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea.
- KMID: 2368488
- DOI: http://doi.org/10.5217/ir.2017.15.1.75
Abstract
- BACKGROUND/AIMS
Cyclooxygenase-2 (COX-2), 15-hydroxyprostaglandin dehydrogenase (15-PGDH), and microsomal prostaglandin E synthase-1 (mPGEs-1) regulate prostaglandin Eâ‚‚ (PGEâ‚‚) expression and are involved in colon carcinogenesis. We investigated the expression of PGEâ‚‚ and its regulating genes in sporadic human colon tumors and matched normal tissues.
METHODS
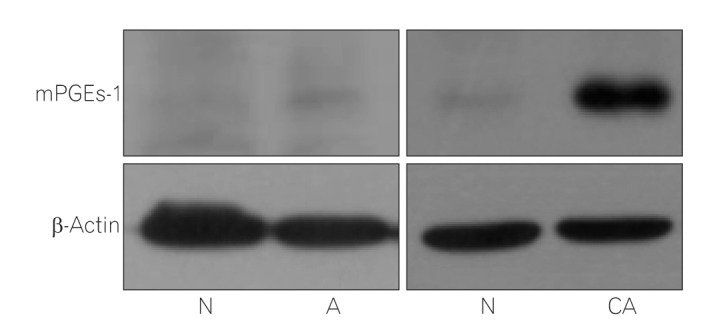
Twenty colonic adenomas and 27 colonic adenocarcinomas were evaluated. COX-2 and 15-PGDH expression was quantified by real-time polymerase chain reaction. The expression of PGEâ‚‚ and mPGEs-1 was measured using enzyme-linked immunosorbent assay and Western blotting, respectively.
RESULTS
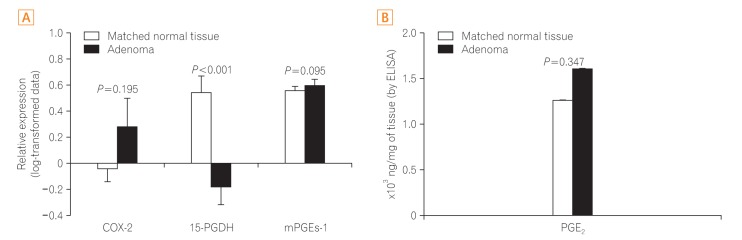
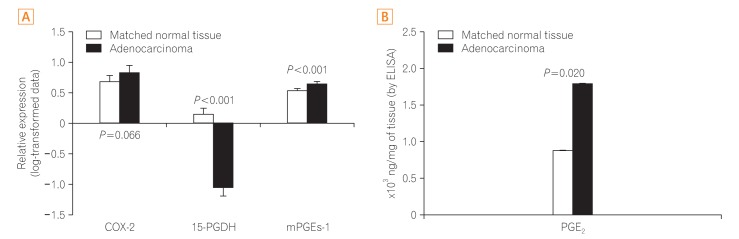
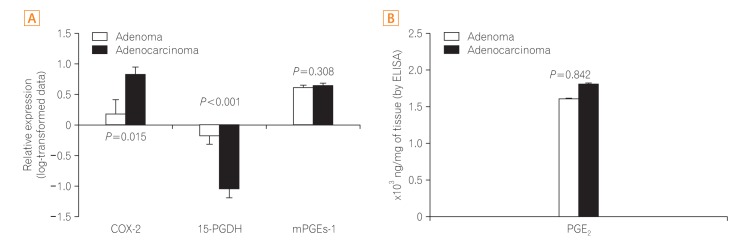
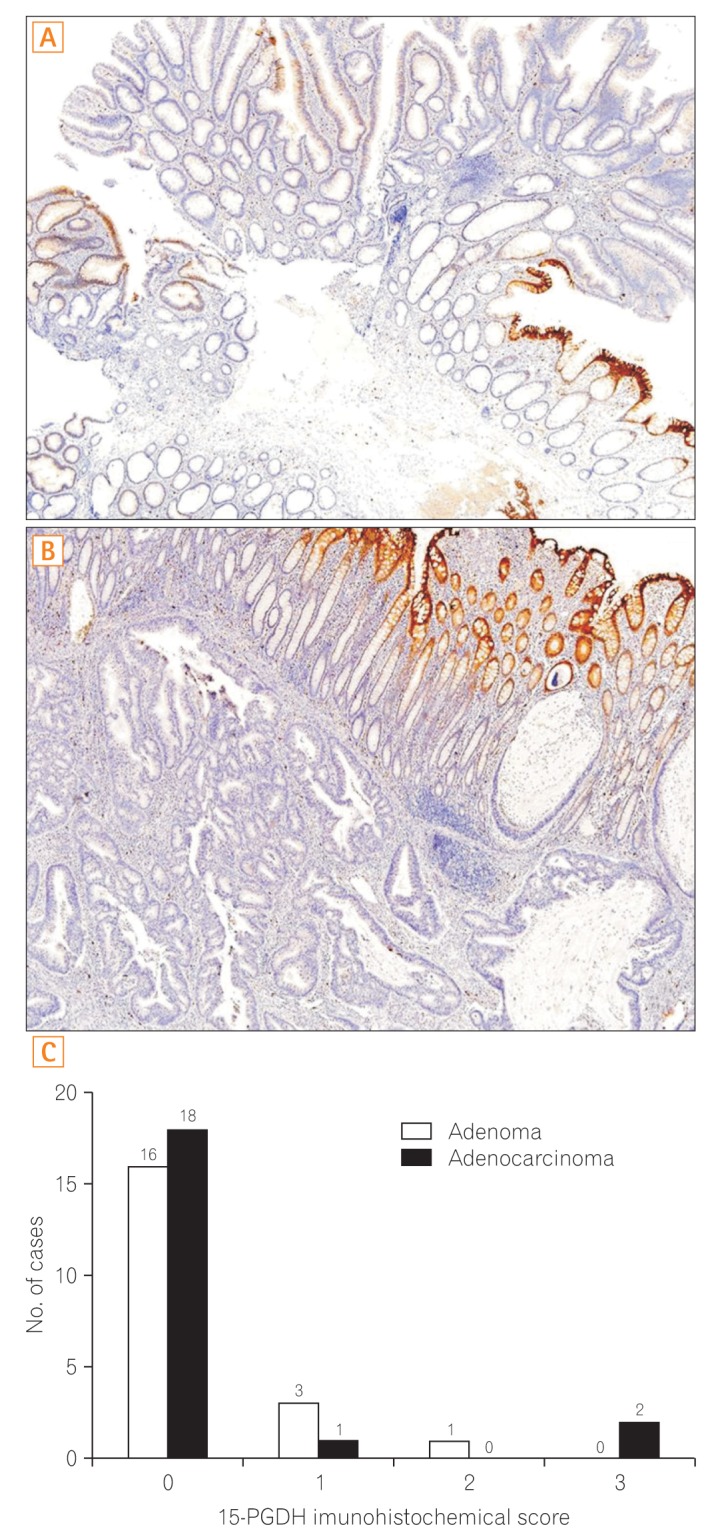
The expression of COX-2, mPGEs-1, and PGEâ‚‚ did not differ between the adenomas and matched distant normal tissues. 15-PGDH expression was lower in adenomas than in the matched normal colonic tissues (P<0.001). In adenocarcinomas, mPGEs-1 and PGEâ‚‚ expression was significantly higher (P<0.001 and P=0.020, respectively), and COX-2 expression did not differ from that in normal tissues (P=0.207). 15-PGDH expression was significantly lower in the normal colonic mucosa from adenocarcinoma patients than in the normal mucosa from adenoma patients (P=0.018).
CONCLUSIONS
Early inactivation of 15-PGDH, followed by activation of COX-2 and mPGEs-1, contributes to PGEâ‚‚ production, leading to colon carcinogenesis. 15-PGDH might be a novel candidate marker for early detection of field defects in colon carcinogenesis.
MeSH Terms
Figure
Reference
-
1. Slaughter DP. The role of internal mammary node dissection in the treatment of breast cancer. Proc Inst Med Chic. 1953; 19:300.2. Cheung DY, Kim TH, Kim CW, et al. The anatomical distribution of colorectal cancer in Korea: evaluation of the incidence of proximal and distal lesions and synchronous adenomas. Intern Med. 2008; 47:1649–1654. PMID: 18827411.
Article3. Shin A, Hong CW, Sohn DK, et al. Associations of cigarette smoking and alcohol consumption with advanced or multiple colorectal adenoma risks: a colonoscopy-based case-control study in Korea. Am J Epidemiol. 2011; 174:552–562. PMID: 21791710.
Article4. Jothy S, Slesak B, Harłozińska A, Lapińska J, Adamiak J, Rabczyński J. Field effect of human colon carcinoma on normal mucosa: relevance of carcinoembryonic antigen expression. Tumour Biol. 1996; 17:58–64. PMID: 7501974.
Article5. Chen LC, Hao CY, Chiu YS, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004; 64:3694–3700. PMID: 15150130.
Article6. Shen L, Kondo Y, Rosner GL, et al. MGMT promoter methylation and field defect in sporadic colorectal cancer. J Natl Cancer Inst. 2005; 97:1330–1338. PMID: 16174854.
Article7. Müller-Decker K, Fürstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog. 2007; 46:705–710. PMID: 17546626.
Article8. Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005; 310:1504–1510. PMID: 16293724.
Article9. Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005; 65:1822–1829. PMID: 15753380.
Article10. Dannenberg AJ, Altorki NK, Boyle JO, et al. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001; 2:544–551. PMID: 11905709.
Article11. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994; 107:1183–1188. PMID: 7926468.
Article12. Myung SJ, Rerko RM, Yan M, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006; 103:12098–12102. PMID: 16880406.
Article13. Backlund MG, Mann JR, Holla VR, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005; 280:3217–3223. PMID: 15542609.
Article14. Lee HJ, Yang DH, Ryu YM, et al. 15-Hydroxyprostaglandin dehydrogenase in colorectal mucosa as a potential biomarker for predicting colorectal neoplasms. J Korean Med Sci. 2013; 28:1154–1160. PMID: 23960441.
Article15. Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002; 68-69:483–493. PMID: 12432938.
Article16. Yan M, Rerko RM, Platzer P, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004; 101:17468–17473. PMID: 15574495.
Article17. Ryu YM, Myung SJ, Park YS, et al. Inhibition of 15-hydroxyprostaglandin dehydrogenase by Helicobacter pylori in human gastric carcinogenesis. Cancer Prev Res (Phila). 2013; 6:349–359. PMID: 23430757.
Article18. Yoshimatsu K, Golijanin D, Paty PB, et al. Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin Cancer Res. 2001; 7:3971–3976. PMID: 11751489.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Prostaglandins in Colon Cancer
- Upregulation of prostaglandin E2 by inducible microsomal prostaglandin E synthase-1 in colon cancer
- Decreased Expression of 15-hydroxyprostaglandin Dehydrogenase in Gastric Carcinomas
- 15-Deoxy-Δ(12,14)-prostaglandin J₂ Upregulates the Expression of 15-Hydroxyprostaglandin Dehydrogenase by Inducing AP-1 Activation and Heme Oxygenase-1 Expression in Human Colon Cancer Cells
- Erratum: 15-Deoxy-(12,14)-prostaglandin Jâ‚‚ Upregulates the Expression of 15-Hydroxyprostaglandin Dehydrogenase by Inducing AP-1 Activation and Heme Oxygenase-1 Expression in Human Colon Cancer Cells