J Korean Med Sci.
2013 Aug;28(8):1154-1160. 10.3346/jkms.2013.28.8.1154.
15-Hydroxyprostaglandin Dehydrogenase in Colorectal Mucosa as a Potential Biomarker for Predicting Colorectal Neoplasms
- Affiliations
-
- 1Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sjmyung@amc.seoul.kr
- 2Asan Digestive Disease Research Institute and Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 1793019
- DOI: http://doi.org/10.3346/jkms.2013.28.8.1154
Abstract
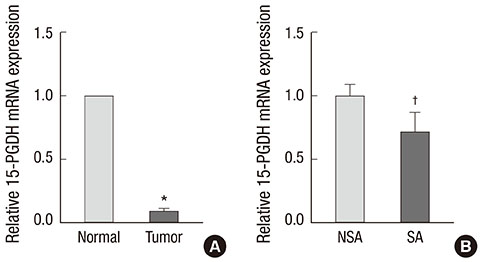
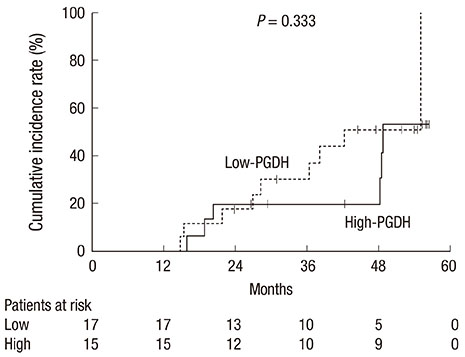
- 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) is downregulated during the early stages of colorectal carcinogenesis. The aim of the present study was to investigate the potential role of 15-PGDH in normal-appearing colorectal mucosa as a biomarker for predicting colorectal neoplasms. We obtained paired tumor and normal tissues from the surgical specimens of 32 sporadic colorectal cancer patients. mRNA expression of 15-PGDH was measured using a quantitative real-time PCR assay. We evaluated the association between 15-PGDH mRNA expression in normal-appearing mucosa, the presence of synchronous adenoma, and the cumulative incidence of metachronous adenoma. The relative 15-PGDH expression of normal-appearing mucosa in patients with synchronous adenoma was significantly lower than in patients without synchronous adenoma (0.71 vs 1.00, P = 0.044). The patients in the lowest tertile of 15-PGDH expression in normal-appearing mucosa were most likely to have synchronous adenoma (OR: 10.5, P = 0.024). Patients with low 15-PGDH expression in normal-appearing mucosa also demonstrated more advanced stage colorectal cancer (P = 0.045). However, there was no significant difference in the cumulative incidence of metachronous adenoma according to 15-PGDH mRNA expression in normal-appearing mucosa (P = 0.333). Hence, 15-PGDH in normal-appearing colorectal mucosa can be a useful biomarker of field effect for the prediction of sporadic synchronous neoplasms.
MeSH Terms
-
Aged
Colorectal Neoplasms/*diagnosis/enzymology/pathology
Down-Regulation
Female
Humans
Hydroxyprostaglandin Dehydrogenases/genetics/*metabolism
Intestinal Mucosa/*enzymology
Male
Middle Aged
Neoplasm Staging
Neoplasms, Multiple Primary/enzymology/pathology
Neoplasms, Second Primary/enzymology/pathology
Odds Ratio
Predictive Value of Tests
RNA, Messenger/metabolism
Real-Time Polymerase Chain Reaction
Risk Factors
Tumor Markers, Biological/*metabolism
Hydroxyprostaglandin Dehydrogenases
RNA, Messenger
Tumor Markers, Biological
Figure
Cited by 1 articles
-
15-Hydroxyprostaglandin dehydrogenase as a marker in colon carcinogenesis: analysis of the prostaglandin pathway in human colonic tissue
Dong-Hoon Yang, Yeon-Mi Ryu, Sun-Mi Lee, Jin-Yong Jeong, Soon Man Yoon, Byong Duk Ye, Jeong-Sik Byeon, Suk-Kyun Yang, Seung-Jae Myung
Intest Res. 2017;15(1):75-82. doi: 10.5217/ir.2017.15.1.75.
Reference
-
1. Markowitz SD. Aspirin and colon cancer: targeting prevention? N Engl J Med. 2007; 356:2195–2198.2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.3. Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009; 24:995–1003.4. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009; 361:2449–2460.5. Srivastava S, Verma M, Henson DE. Biomarkers for early detection of colon cancer. Clin Cancer Res. 2001; 7:1118–1126.6. Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007; 58:239–252.7. Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001; 1:11–21.8. Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002; 68-69:483–493.9. Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G, et al. 15-hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006; 103:12098–12102.10. Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, Lawrence E, Lutterbaugh J, Lu S, Willson JK, et al. 15-hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004; 101:17468–17473.11. Smartt HJ, Greenhough A, Ordóñez-Morán P, Talero E, Cherry CA, Wallam CA, Parry L, Al Kharusi M, Roberts HR, Mariadason JM, et al. β-catenin represses expression of the tumour suppressor 15-prostaglandin dehydrogenase in the normal intestinal epithelium and colorectal tumour cells. Gut. 2012; 61:1306–1314.12. Jothy S, Slesak B, Harłozinska A, Lapinska J, Adamiak J, Rabczynski J. Field effect of human colon carcinoma on normal mucosa: relevance of carcinoembryonic antigen expression. Tumour Biol. 1996; 17:58–64.13. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010; 138:2029–2043.e10.14. Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993; 260:816–819.15. Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter's concept of field cancerization evidence and clinical implications. Cancer Res. 2003; 63:1727–1730.16. Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953; 6:963–968.17. Shen L, Kondo Y, Rosner GL, Xiao L, Hernandez NS, Vilaythong J, Houlihan PS, Krouse RS, Prasad AR, Einspahr JG, et al. MGMT promoter methylation and field defect in sporadic colorectal cancer. J Natl Cancer Inst. 2005; 97:1330–1338.18. Belshaw NJ, Pal N, Tapp HS, Dainty JR, Lewis MP, Williams MR, Lund EK, Johnson IT. Patterns of DNA methylation in individual colonic crypts reveal aging and cancer-related field defects in the morphologically normal mucosa. Carcinogenesis. 2010; 31:1158–1163.19. Worthley DL, Whitehall VL, Buttenshaw RL, Irahara N, Greco SA, Ramsnes I, Mallitt KA, Le Leu RK, Winter J, Hu Y, et al. DNA methylation within the normal colorectal mucosa is associated with pathwayspecific predisposition to cancer. Oncogene. 2010; 29:1653–1662.20. Keku TO, Amin A, Galanko J, Martin C, Schliebe B, Sandler RS. Apoptosis in normal rectal mucosa, baseline adenoma characteristics, and risk of future adenomas. Cancer Epidemiol Biomarkers Prev. 2008; 17:306–310.21. Martin C, Connelly A, Keku TO, Mountcastle SB, Galanko J, Woosley JT, Schliebe B, Lund PK, Sandler RS. Nonsteroidal anti-inflammatory drugs, apoptosis, and colorectal adenomas. Gastroenterology. 2002; 123:1770–1777.22. Baytner S, Mitmaker B, Gordon PH, Wang E. Immunohistochemical expression of mutant p53 oncogene in transitional mucosa adjacent to human colon cancer. Clin Invest Med. 1993; 16:379–385.23. Chung SJ, Kim YS, Yang SY, Song JH, Kim D, Park MJ, Kim SG, Song IS, Kim JS. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011; 60:1537–1543.24. Martínez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology. 2001; 120:1077–1083.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Implication of 15-Hydroxyprostaglandin Dehydrogenase Down-Regulation in Patients with Colorectal Cancer
- Role of Prostaglandins in Colon Cancer
- Expression of Tumor Metastasis Related Genes in Korean Colorectal Cancers and Cell lines
- Decreased Expression of 15-hydroxyprostaglandin Dehydrogenase in Gastric Carcinomas
- Finding a New Prognostic Biomarker for Metastatic Colorectal Cancer