Ann Lab Med.
2013 Mar;33(2):111-115. 10.3343/alm.2013.33.2.111.
Surveillance of Antimicrobial Susceptibility Patterns among Shigella Species Isolated in China during the 7-Year Period of 2005-2011
- Affiliations
-
- 1Department of Infectious Diseases, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China. lijiabin948@vip.sohu.com
- 2Institute of Bacterial Resistance, Anhui Medical University, Hefei, Anhui, China.
- 3Anhui Center for Surveillance of Bacterial Resistance, Hefei, Anhui, China.
- KMID: 1781309
- DOI: http://doi.org/10.3343/alm.2013.33.2.111
Abstract
- BACKGROUND
Shigella is a frequent cause of bacterial dysentery in the developing world. Treatment with antibiotics is recommended for shigellosis, but the options are limited due to globally emerging resistance. This study was conducted to determine the frequency and pattern of antimicrobial susceptibility of Shigella in China.
METHODS
We studied the antimicrobial resistance profiles of 308 Shigella spp. strains (260 S. flexneri, 40 S. sonnei, 5 S. boydii, and 3 S. dysenteriae) isolated from fecal samples of patients (age, from 3 months to 92 yr) presenting with diarrhea in different districts of Anhui, China. The antimicrobial resistance of strains was determined by the agar dilution method according to the CSLI guidelines.
RESULTS
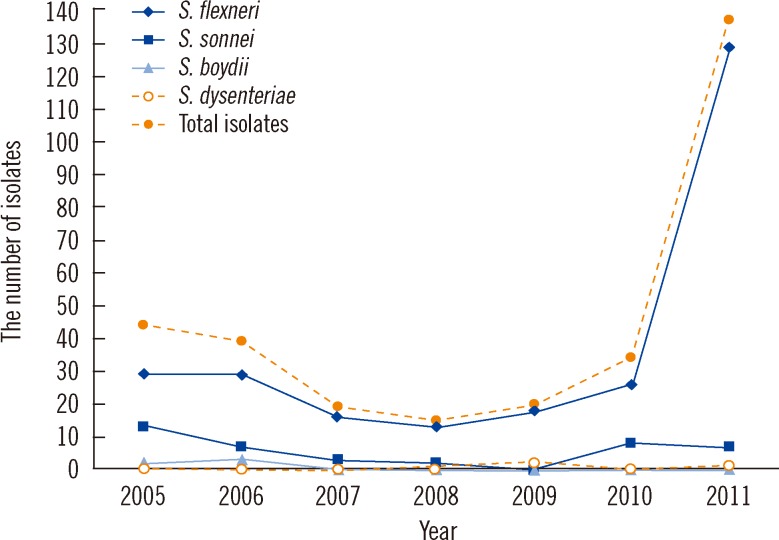
The most common serogroup in the Shigella isolates was S. flexneri (n=260, 84.4%), followed by S. sonnei (n=40, 13.0%). The highest resistance rate was found for nalidixic acid (96.4%), followed by ampicillin (93.2%), tetracycline (90.9%), and trimethoprim/sulfamethoxazole (80.8%). Among the isolates tested, 280 (91.0%) were multidrug resistant (resistant to > or =2 agents). The most common resistance pattern was the combination of ampicillin, tetracycline, and trimethoprim/sulfamethoxazole (70.8%). Resistance to ampicillin and tetracycline were more common among S. flexneri than among S. sonnei isolates.
CONCLUSIONS
S. flexneri is predominant in Anhui, China, and its higher antimicrobial resistance rate compared with that of S. sonnei is a cause for concern. Continuous monitoring of resistance patterns is necessary to control the spread of resistance in Shigella. The recommendations for antimicrobial treatment must be updated regularly based on surveillance results.
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Ampicillin/pharmacology
Anti-Infective Agents/*pharmacology
Child
Child, Preschool
China
Drug Resistance, Bacterial/drug effects
Dysentery, Bacillary/*diagnosis/microbiology
Feces/microbiology
Humans
Infant
Microbial Sensitivity Tests
Middle Aged
Nalidixic Acid/pharmacology
Shigella/*drug effects/isolation & purification
Shigella flexneri/drug effects/isolation & purification
Shigella sonnei/drug effects/isolation & purification
Tetracycline/pharmacology
Time Factors
Trimethoprim-Sulfamethoxazole Combination/pharmacology
Young Adult
Anti-Infective Agents
Nalidixic Acid
Tetracycline
Ampicillin
Trimethoprim-Sulfamethoxazole Combination
Figure
Cited by 1 articles
-
Infection of Extended-Spectrum β-Lactamase Producing Shigella flexneri in Children Attending a Childcare Center in Korea
Eun Woo Nam, Kun Song Lee, Junyoung Kim, Cheon Kwon Yoo
Pediatr Infect Vaccine. 2016;23(3):223-228. doi: 10.14776/piv.2016.23.3.223.
Reference
-
1. Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999; 77:651–666. PMID: 10516787.2. Legros D, editor. Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. 2005. Geneva: World Health Organization;http://www.who.int/vaccine_research/documents/Guidelines_Shigellosis.pdf.3. Sur D, Ramamurthy T, Deen J, Bhattacharya SK. Shigellosis: challenges & management issues. Indian J Med Res. 2004; 120:454–462. PMID: 15591629.4. Wang XY, Tao F, Xiao D, Lee H, Deen J, Gong J, et al. Trend and disease burden of bacillary dysentery in China (1991-2000). Bull World Health Organ. 2006; 84:561–568. PMID: 16878230.
Article5. Bhattacharya SK, Sur D. An evaluation of current shigellosis treatment. Expert Opin Pharmacother. 2003; 4:1315–1320. PMID: 12877639.
Article6. Watanabe T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963; 27:87–115. PMID: 13999115.
Article7. Sack RB, Rahman M, Yunus M, Khan EH. Antimicrobial resistance in organisms causing diarrheal disease. Clin Infect Dis. 1997; 24:S102–S105. PMID: 8994788.
Article8. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Twenty-second Informational supplement, M100-S22. 2012. Wayne, PA: Clinical and Laboraotory Standards Institute.9. Sivapalasingam S, Nelson JM, Joyce K, Hoekstra M, Angulo FJ, Mintz ED. High prevalence of antimicrobial resistance among Shigella isolates in the United States tested by the National Antimicrobial Resistance Monitoring System from 1999 to 2002. Antimicrob Agents Chemother. 2006; 50:49–54. PMID: 16377666.10. Ashkenazi S, May-Zahav M, Dinari G, Gabbay U, Zilberberg R, Samra Z. Recent trends in the epidemiology of Shigella species in Israel. Clin Infect Dis. 1993; 17:897–899. PMID: 8286636.
Article11. DeLappe N, O'Halloran F, Fanning S, Corbett-Feeney G, Cheasty T, Cormican M. Antimicrobial resistance and genetic diversity of Shigella sonnei isolates from western Ireland, an area of low incidence of infection. J Clin Microbiol. 2003; 41:1919–1924. PMID: 12734227.12. Ashkenazi S, Levy I, Kazaronovski V, Samra Z. Growing antimicrobial resistance of Shigella isolates. J Antimicrob Chemother. 2003; 51:427–429. PMID: 12562716.
Article13. von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, et al. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 2006; 3:e353. PMID: 16968124.
Article14. Gu B, Cao Y, Pan S, Zhuang L, Yu R, Peng Z, et al. Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe-America and Asia-Africa from 1998 to 2009. Int J Antimicrob Agents. 2012; 40:9–17. PMID: 22483324.
Article15. Xia S, Xu B, Huang L, Zhao JY, Ran L, Zhang J, et al. Prevalence and characterization of human Shigella infections in Henan Province, China, in 2006. J Clin Microbiol. 2011; 49:232–242. PMID: 21068291.
Article16. Pu XY, Pan JC, Wang HQ, Zhang W, Huang ZC, Gu YM. Characterization of fluoroquinolone-resistant Shigella flexneri in Hangzhou area of China. J Antimicrob Chemother. 2009; 63:917–920. PMID: 19297378.
Article17. Xiong Z, Li J, Li T, Shen J, Hu F, Wang M. Prevalence of plasmid-mediated quinolone-resistance determinants in Shigella flexneri isolates from Anhui Province, China. J Antibiot (Tokyo). 2010; 63:187–189. PMID: 20203702.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial Resistance Pattern and Ribotyping of Shigella sonnei Isolated in Korea
- Trends in Isolation and Antimicrobial Susceptibility of Enteropathogenic Bacteria in 2011-2019 at a Korean Tertiary Care Hospital Compared with Data in the Preceding Reports
- Asymptomatic Urinary Tract Infection Caused by Shigella sonnei
- Species and Antimicrobial Susceptibilities of Bacteria Isolated at Dankook University Hospital in 1994-1996
- Epidemiological Characterization of Shigella flexneri Isolates in Korea and the Analysis of Pulsed - Field Gel Electrophoresis Patterns