J Korean Med Sci.
2010 Feb;25(2):265-271. 10.3346/jkms.2010.25.2.265.
Eradication of Helicobacter pylori Increases Ghrelin mRNA Expression in the Gastric Mucosa
- Affiliations
-
- 1Department of Family Medicine, Center for Health Promotion and Clinical Research Center, Ilsan-Paik Hospital, College of Medicine, Inje University, Goyang, Korea.
- 2Department of Endocrinology, Kangbuk Samsung Hospital, Sungkyunkwan University, School of Medicine, Seoul, Korea.
- 3Department of Pathology, Ilsan-Paik Hospital, College of Medicine, Inje University, Goyang, Korea.
- 4Department of Laboratory Medicine, Ilsan-Paik Hospital, College of Medicine, Inje University, Goyang, Korea.
- 5Department of Gastroenterology, Bundang Jesaeng Hospital, Seongnam, Korea.
- 6Center for Obesity, Nutrition and Metabolism, Department of Family Medicine, Pusan National University Hospital Medical Research Institute, Pusan National University, Medical Education Unit, Pusan National University School of Medicine, Busan, Korea.
- 7Department of Family Medicine, Center for Obesity, Metabolism and Nutrition, College of Medicine, Dongguk University Ilsan Hospital, Goyang, Korea. osw6021@yahoo.co.kr
- KMID: 1779258
- DOI: http://doi.org/10.3346/jkms.2010.25.2.265
Abstract
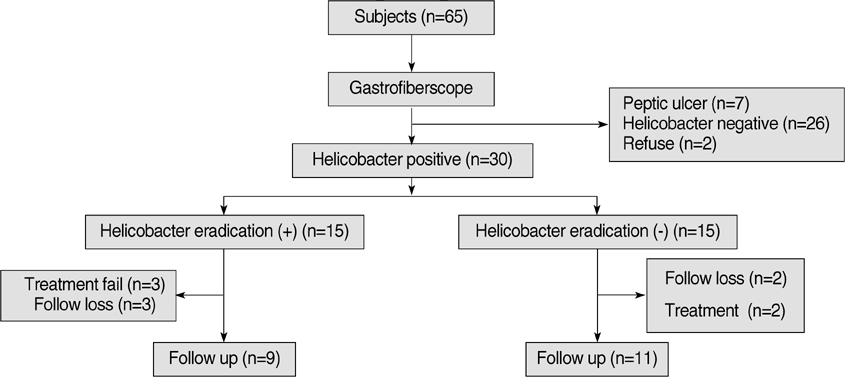
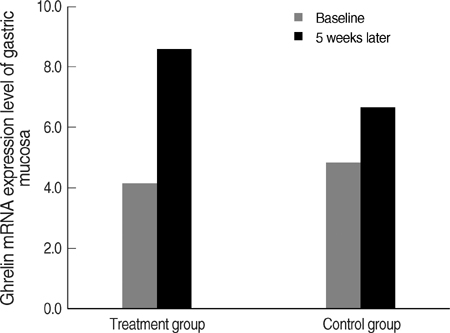
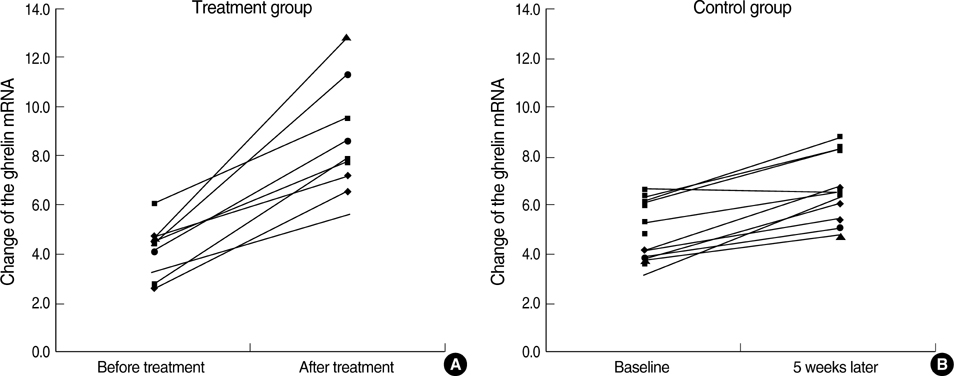
- It has been suggested that Helicobacter pylori eradication may influence production of some peptides in the stomach, which can affect appetite. This hypothesis is controversial. To verify the hypothesis, we conducted this randomized controlled trial using H. pylori infected subjects without any gastrointestinal symptoms. The treatment group received triple H. pylori eradication therapy for 7 days and the control group received no medication. We measured ghrelin, obestatin and the tumor necrosis factor-alpha (TNF-alpha) mRNA levels in endoscopic biopsy specimens and the changes from baseline to follow-up. The plasma active n-octanoyl ghrelin and obestatin levels were measured in both groups. The ghrelin/obestatin ratios in plasma and gastric mRNA expression were calculated at baseline and follow-up. Ghrelin mRNA expression in the fundic mucosa after H. pylori eradication increased significantly compared to the control group (4.47+/-2.14 vs. 1.79+/-0.96, P=0.009), independent of inflammatory changes. However, obestatin mRNA expression decreased in the antral mucosa (-0.57+/-1.06 vs. 0.41+/-0.72, P=0.028). The treatment group showed a marginal increase (P=0.060) in plasma ghrelin/obestatin ratio. The TNF-alpha mRNA expression also decreased significantly with treatment. This randomized controlled trial demonstrates that H. pylori eradication increases ghrelin mRNA expression, independent of inflammatory cell changes.
Keyword
MeSH Terms
-
Adult
Aged
Anti-Bacterial Agents/therapeutic use
Female
Gastric Mucosa/*metabolism/microbiology
Gastroscopy
Ghrelin/blood/genetics/*metabolism
Helicobacter Infections/drug therapy/genetics/*metabolism
*Helicobacter pylori
Humans
Male
RNA, Messenger/metabolism
Tumor Necrosis Factor-alpha/genetics/metabolism
Anti-Bacterial Agents
Ghrelin
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984. 1:1311–1315.
Article2. Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007. 133:659–672.
Article3. Bravo LE, Mera R, Reina JC, Pradilla A, Alzate A, Fontham E, Correa P. Impact of Helicobacter pylori infection on growth of children: a prospective cohort study. J Pediatr Gastroenterol Nutr. 2003. 37:614–619.
Article4. Cho I, Blaser MJ, Francois F, Mathew JP, Ye XY, Goldberg JD, Bini EJ. Helicobacter pylori and overweight status in the United States: data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2005. 162:579–584.
Article5. Azuma T, Suto H, Ito Y, Muramatsu A, Ohtani M, Dojo M, Yamazaki Y, Kuriyama M, Kato T. Eradication of Helicobacter pylori infection induces an increase in body mass index. Aliment Pharmacol Ther. 2002. 16:Suppl 2. 240–244.
Article6. Furuta T, Shirai N, Xiao F, Takashima M, Hanai H. Effect of Helicobacter pylori infection and its eradication on nutrition. Aliment Pharmacol Ther. 2002. 16:799–806.
Article7. Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005. 85:495–522.
Article8. St-Pierre DH, Wang L, Tache Y. Ghrelin: a novel player in the gut brain regulation of growth hormone and energy balance. News Physiol Sci. 2003. 18:242–246.9. Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001. 86:5992.
Article10. Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, Shiiya T, Satoh K, Ishino Y, Sugano K. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab. 2005. 90:10–16.11. Nwokolo CU, Freshwater DA, O'Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003. 52:637–640.
Article12. Tatsuguchi A, Miyake K, Gudis K, Futagami S, Tsukui T, Wada K, Kishida T, Fukuda Y, Sugisaki Y, Sakamoto C. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol. 2004. 99:2121–2127.
Article13. Shinomiya T, Fukunaga M, Akamizu T, Irako T, Yokode M, Kangawa K, Nakai Y, Nakai Y. Plasma acylated ghrelin levels correlate with subjective symptoms of functional dyspepsia in female patients. Scand J Gastroenterol. 2005. 40:648–653.
Article14. Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005. 310:996–999.
Article15. Guo ZF, Zheng X, Qin YW, Hu JQ, Chen SP, Zhang Z. Circulating preprandial ghrelin to obestatin ratio is increased in human obesity. J Clin Endocrinol Metab. 2007. 92:1875–1880.
Article16. Huda MS, Durham BH, Wong SP, Deepak D, Kerrigan D, McCulloch P, Ranganath L, Pinkney J, Wilding JP. Plasma obestatin levels are lower in obese and post-gastrectomy subjects, but do not change in response to a meal. Int J Obes (Lond). 2008. 32:129–135.
Article17. Jang EJ, Park SW, Park JS, Park SJ, Hahm KB, Paik SY, Sin MK, Lee ES, Oh SW, Park CY, Baik HW. The influence of the eradication of Helicobacter pylori on gastric ghrelin, appetite, and body mass index in patients with peptic ulcer disease. J Gastroenterol Hepatol. 2008. 23:Suppl 2. S278–S285.18. Isomoto H, Ueno H, Nishi Y, Wen CY, Nakazato M, Kohno S. Impact of Helicobacter pylori infection on ghrelin and various neuroendocrine hormones in plasma. World J Gastroenterol. 2005. 11:1644–1648.19. Kim HB, Lee ES, Oh SW, Kim YH, Lee DE, Hwang CK, Lee EY, Yoon YS, Yang YJ. Validity, reproducibility of visual analogue scales in assessment of appetite sensations. J Korean Acad Fam Med. 2008. 29:736–745.20. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996. 20:1161–1181.21. Konturek PC, Cześnikiewicz-Guzik M, Bielanski W, Konturek SJ. Involvement of Helicobacter pylori infection in neuro-hormonal control of food intake. J Physiol Pharmacol. 2006. 57:Suppl 5. 67–81.22. Moayyedi P, Soo S, Deeks J, Delaney B, Harris A, Innes M, Oakes R, Wilson S, Roalfe A, Bennett C, Forman D. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006. 2:CD002096.
Article23. Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, Ohnita K, Mizuta Y, Ohtsuru A, Yamashita S, Nakazato M, Kohno S. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol. 2005. 100:1711–1720.
Article24. Cummings DE. Helicobacter pylori and ghrelin: interrelated players in body-weight regulation? Am J Med. 2004. 117:436–439.
Article25. Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004. 113:321–333.
Article26. Bresciani E, Rapetti D, Donà F, Bulgarelli I, Tamiazzo L, Locatelli V, Torsello A. Obestatin inhibits feeding but does not modulate GH and corticosterone secretion in the rat. J Endocrinol Invest. 2006. 29:RC16–RC18.
Article27. Zizzari P, Longchamps R, Epelbaum J, Bluet-Pajot MT. Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology. 2007. 148:1648–1653.
Article28. Nogueiras R, Pfluger P, Tovar S, Arnold M, Mitchell S, Morris A, Perez-Tilve D, Vázquez MJ, Wiedmer P, Castañeda TR, DiMarchi R, Tschöp M, Schurmann A, Joost HG, Williams LM, Langhans W, Diéguez C. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology. 2007. 148:21–26.
Article29. Seoane LM, Al-Massadi O, Pazos Y, Pagotto U, Casanueva FF. Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats. J Endocrinol Invest. 2006. 29:RC13–RC15.
Article30. Vicennati V, Genghini S, De Iasio R, Pasqui F, Pagotto U, Pasquali R. Circulating obestatin levels and the ghrelin/obestatin ratio in obese women. Eur J Endocrinol. 2007. 157:295–301.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ghrelin Levels in Gastric Mucosa before and after Eradication of Helicobacter pylori
- Effectiveness of Helicobacter pylori Eradication before Endoscopic Resection
- Expression of CCL18 (Dendritic Cell-Derived Chemokine) mRNA in Gastric Mucosa Infected with Helicobacter pylori
- Prevention of Gastric Cancer: Helicobacter pylori Treatment
- The Expression of Human beta-defensin 2 in Helicobacter pylori Infection