J Korean Med Sci.
2005 Jun;20(3):456-460. 10.3346/jkms.2005.20.3.456.
Identification of New Proteins in Follicular Fluid from Mature Human Follicles by Direct Sample Rehydration Method of Two-Dimensional Polyacrylamide Gel Electrophoresis
- Affiliations
-
- 1Functional Genomics Lab, CHA Research Institute, Bundang Campus, College of Medicine, Pochon CHA University, Sungnam, Korea. suman@cha.ac.kr
- 2Genome Research Center for Reproductive Medicine and Infertility, CHA General Hospital, Seoul, Korea.
- 3Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1778506
- DOI: http://doi.org/10.3346/jkms.2005.20.3.456
Abstract
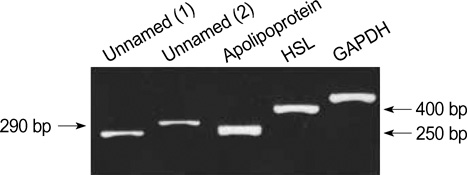
- Human follicular fluid (HFF) includes various biologically active proteins which can affect follicle growth and oocyte fertilization. Thus far, these proteins from mature follicles in human follicular fluid have been poorly characterized. Here, two-dimensional polyacrylamide gel electrophoresis (2-DE) with matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS) was used to identify new proteins in HFF. Mature follicular fluids were obtained from five females after oocyte collection during in vitro fertilization (IVF). We directly rehydrated HFF samples, obtained high-resolution 2-DE maps, and processed them for 2-DE and MALDI-MS. One hundred eighty spots were detected and 10 of these spots were identified. By the 2-DE database, six of them had been reported, as proteins already existing in HFF. Hormone sensitive lipase (HSL), Unnamed protein product 1 (UPP1), Unnamed protein product 2 (UPP2), and apolipoprotein A-IV precursor were newly detected. HSL and apolipoprotein A-IV participate in lipid metabolism. UPP1 has a homology with selenocysteine lyase. We found by RT-PCR that these genes are expressed from human primary granulosa cells. The proteins identified here may emerge as potential candidates for specific functions during folliculogenesis, hormone secretion regulation, or oocyte maturation. Further functional analysis of these proteins is necessitated to determine their biological implications.
MeSH Terms
-
Adult
Electrophoresis, Gel, Two-Dimensional/*methods
Female
Follicular Fluid/*chemistry/metabolism
Gene Expression
Granulosa Cells/metabolism
Humans
Ovarian Follicle/*chemistry/metabolism
Proteins/*analysis/genetics
RNA, Messenger/genetics/metabolism
Research Support, Non-U.S. Gov't
Reverse Transcriptase Polymerase Chain Reaction
Spectrometry, Mass, Matrix-Assisted Laser Desorption-Ionization
Figure
Reference
-
1. Nayudu PL, Lopata A, Jones GM, Gook DA, Bourne HM, Sheather SJ, Brown TC, Johnston WI. An analysis of human oocytes and follicles from stimulated cycles: oocyte morphology and associated follicular fluid characteristics. Hum Reprod. 1989. 4:558–567.2. Kawano Y, Fukuda J, Nasu K, Nishida M, Narahara H, Miyakawa I. Production of macrophage inflammatory protein-α in human follicular fulid and culture egranulosa cells. Fertil Steril. 2004. 82:1206–1211.3. Ocal P, Aydin S, Cepni I, Idil S, Idil M, Uzun H, Benian A. Follicular fluid concentrations of vascular endothelial growth factor, inhibin A and inhibin B in IVF cycles: are they markers for ovarian response and pregnancy outcome? Eur J Obstet Gynecol Reprod Biol. 2004. 115:194–199.
Article4. Cunha-Filho JS, Lemos NA, Freitas FM, Kiefer K, Faller M, Passos EP. Insulin-like growth factor (IGF)-1 and IGF binding protein-1 and -3 in the follicular fluid of infertile patients with endometriosis. Hum Reprod. 2003. 18:423–428.
Article5. Hong SJ, Tse JY, Ho PC, Yeung WS. Cumulus cells reduce the spermatozoa-zona binding inhibitory activity of human follicular fluid. Fertil Steril. 2003. 79:802–807.6. Anderson NG, Anderson NL. Twenty years of two-dimensional electrophoresis: past, present and future. Electrophoresis. 1996. 17:443–453.
Article7. Washburn MP, Wolters D, Yates JR III. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001. 19:242–247.
Article8. Ryu JW, Kim HJ, Lee YS, Myong NH, Hwang CH, Lee GS, Yom HC. The proteomic approach to find biomarkers in gastric cancer. J Korean Med Sci. 2003. 18:505–509.9. Fryksdale BG, Jedrzejewski PT, Wong DL, Gaerther AL, Miller BS. Impact of de-glycosylation methods on two-dimensional gel electrophoresis and matrix assisted laser desorption/ionization-time of flight-mass spectrometry for proteomic analysis. Electrophoresis. 2002. 23:2184–2193.
Article10. Anahory T, Dechaud H, Bennes R, Marin P, Lamb NJ, Laoudj D. Identification of new proteins in follicular fluid of mature human follicles. Electrophoresis. 2002. 23:1197–1202.
Article11. Hughes GJ, Frutiger S, Paquet N, Ravier F, Pasquali C, Sanchez JC, James R, Tissot JD, Bjellqvist , Hochstrasser DF. Plasma protein map: an update by microsequencing. Electrophoresis. 1992. 13:707–714.
Article12. Abdullaev FI, MacVicar C, Frenkel GD. Inhibition by selenium of DNA and RNA synthesis in normal and malignant human cells in vitro. Cancer Lett. 1992. 65:43–49.
Article13. Spyrou G, Bjornstedt M, Kumar S, Holmgren A. AP-1 DNA-binding activity is inhibited by selenite and selenodiglutathione. FEBS Lett. 1995. 368:59–63.
Article14. Sinha R, Medina D. Inhibition of cdk2 kinase activity by methylselenocysteine in synchronized mouse mammary epithelial tumor cells. Carcinogenesis. 1997. 18:1541–1547.
Article15. Fiala ES, Staretz ME, Pandya GA, El-Bayoumy K, Hamilton SR. Inhibition of DNA cytosine methyltransferase by chemopreventive selenium compounds, determined by an improved assay for DNA cytosine methyltransferase and DNA cytosine methylation. Carcinogenesis. 1998. 19:597–604.
Article16. Swaney JB, Braithwaite F, Eder HA. Characterization of the apolipoproteins of rat plasma lipoproteins. Biochemistry. 1977. 16:271–278.
Article17. Lefevre M, Roheim PS. Metabolism of apolipoprotein A-IV. J Lipid Res. 1984. 25:1603–1610.
Article18. Shen WJ, Sridhar K, Bernlohr DA, Kraemer FB. Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proc Natl Acad Sci USA. 1999. 96:5528–5532.
Article19. Holm C, Österlund T, Laurell H, Contreras JA. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr. 2000. 20:365–393.
Article20. Londos C, Brasaemle DL, Schultz CJ, Segrest JP, Kimmel AR. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin Cell Dev Biol. 1999. 10:51–58.
Article21. Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol. 2003. 161:1093–1103.
Article22. Faulds G, Ryden M, Ek I, Wahrenberg H, Arner P. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab. 2003. 88:2269–2273.
Article23. Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, Yamada N. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA. 2000. 97:787–792.
Article24. Østerlund T. Structure-function relationships of hormone-sensitive lipase. Eur J Biochem. 2001. 268:1899–1907.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 2DSpotDB: A Database for the Annotated Two-dimensional Polyacrylamide Gel Electrophoresis of Pathogen Proteins
- Identification of Proteome Molecules by Proteomics Using Two-Dimensional Gel Electrophoresis and MALDI-TOF MS
- The study of pathogenesis of palmoplantar keratoderma
- Component proteins in cystic fluid of Taenia solium metacestodes collected surgically from neurocysticercosis patients
- Separation of Tissue proteins in Transitional Cell Carcinoma of Bladder by Two-dimensional Electrophoresis