J Korean Med Sci.
2006 Jun;21(3):500-505. 10.3346/jkms.2006.21.3.500.
Low Dose Radiation Overcomes Diabetes-induced Suppression of Hippocampal Neuronal Cell Proliferation in Rats
- Affiliations
-
- 1Department of Radiation Oncology, School of Medicine, Kyung Hee University, Seoul, Korea. kangjino@khmc.or.kr
- 2Department of Physiology, School of Medicine, Kyung Hee University, Seoul, Korea.
- KMID: 1778436
- DOI: http://doi.org/10.3346/jkms.2006.21.3.500
Abstract
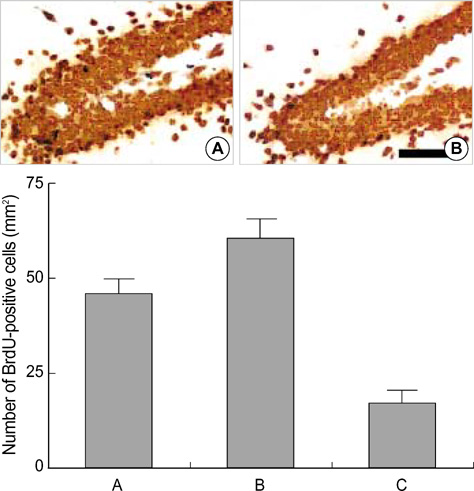
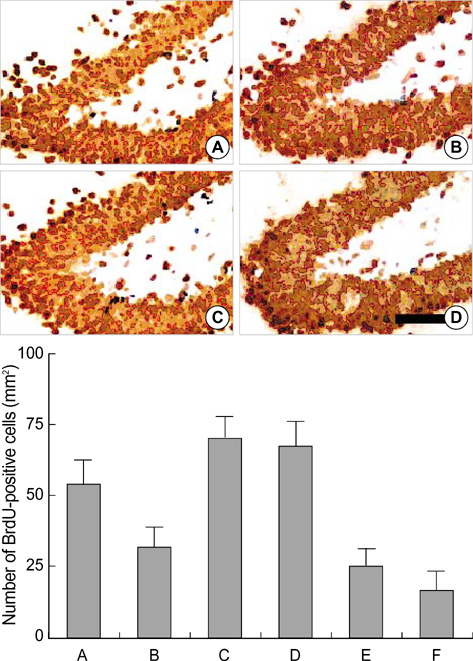
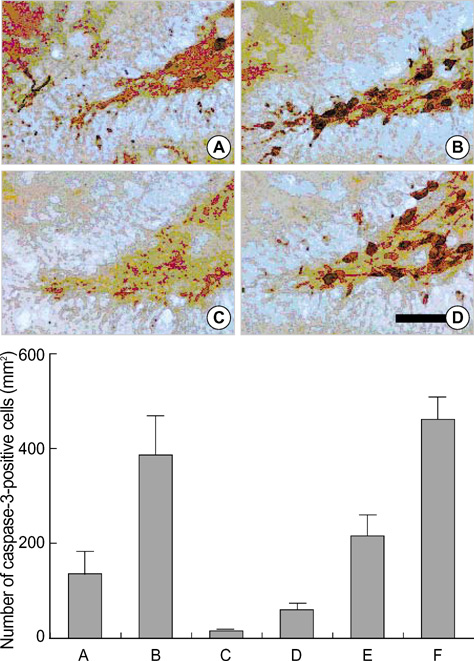
- We investigated the effect of low dose radiation on diabetes induced suppression of neurogenesis in the hippocampal dentate gyrus of rat. After 0.01 Gy, 0.1 Gy, 1 Gy and 10 Gy radiation was delivered, the dentate gyrus of hippocampus of streptozotocin (STZ)-induced diabetic rats were evaluated using immunohistochemistry for 5-bromo-2-deoxyuridine (BrdU), caspase-3, and terminal deoxynucleotidyl transferase-mediated nick end-labeling (TUNEL) staining. The number of BrdU positive cells in the non-diabetic rats, diabetic rats without radiation, diabetic rats with 0.01 Gy radiation, diabetic rats with 0.1 Gy radiation, diabetic rats with 1 Gy radiation and diabetic rats with 10 Gy radiation were 55.4+/-8.5/mm2, 33.3+/-6.4/mm2, 67.7+/-10.5/mm2, 66.6+/-10.0/mm2, 23.5+/-6.3/mm2 and 14.3+/-7.2/mm2, respectively. The number of caspase-3 positive cells was 132.6+/-37.4/mm2, 378.6+/-99.1/mm2, 15.0+/-2.8/mm2, 57.1+/-16.9/mm2, 191.8+/-44.8/mm2 and 450.4+/-58.3/mm2, respectively. The number of TUNEL-positive cells was 24.5+/-2.0/mm2, 21.7+/-4.0/mm2, 20.4+/-2.0/mm2, 18.96+/-2.1/mm2, 58.3+/-7.9/mm2, and 106.0+/-9.8/mm2, respectively. These results suggest low doses of radiation paradoxically improved diabetes induced neuronal cell suppression in the hippocampal dentate gyrus of rat.
Keyword
MeSH Terms
-
Rats, Sprague-Dawley
Rats
Radiotherapy/methods
Neurons/*metabolism
Male
In Situ Nick-End Labeling
Hippocampus/*cytology/metabolism/radiation effects
Diabetes Mellitus, Experimental/radiotherapy
Dentate Gyrus/drug effects/*radiation effects
Cell Proliferation
Caspase 3/metabolism
Bromodeoxyuridine/pharmacology
Apoptosis
Animals
Figure
Cited by 1 articles
-
Adaptive Responses Induced by Low Dose Radiation in Dentate Gyrus of Rats
Jin Oh Kang, Seong Eon Hong, Sang Ki Kim, Chang Ju Kim, Taeck Hyun Lee, Hyun Kyung Chang, Mal Soon Shin, Hong Kim
J Korean Med Sci. 2006;21(6):1103-1107. doi: 10.3346/jkms.2006.21.6.1103.
Reference
-
1. Biessels GJ, Kappelle AC, Kappelle AC, Bravenboer B, Erkelens DW, Gispen WH. Cerebral function in diabetes mellitus. Diabetologia. 1994. 37:643–650.
Article2. Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996. 45:1259–1266.
Article3. Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004. 26:1044–1080.
Article4. Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004. 61:661–666.
Article5. Jackson-Guilford J, Leander JD, Nisenbaum LK. The effect of streptozotocin-induced diabetes on cell proliferation in the rat dentate gyrus. Neurosci Lett. 2000. 293:91–94.
Article6. van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002. 415:1030–1034.
Article7. Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999. 21:46S–51S.
Article8. Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997. 386:493–495.
Article9. Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999. 19:5792–5801.
Article10. Kim YP, Kim HB, Jang MH, Lim BV, Kim YJ, Kim H, Kim SS, Kim EH, Kim CJ. Magnitude- and time-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Int J Sports Med. 2003. 24:114–117.
Article11. Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999. 2:894–897.
Article12. Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999. 46:1472–1479.
Article13. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996. 16:2027–2033.
Article14. Ferrer I, Borras D. Effects of X-irradiation on glial cells in the developing rat brain. Int J Radiat Biol. 1994. 66:181–187.
Article15. Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003. 63:4021–4027.16. Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000. 99:33–41.
Article17. Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003. 16:129–134.
Article18. Shukitt-Hale B, Casadesus G, McEwen JJ, Rabin BM, Joseph JA. Spatial learning and memory deficits induced by exposure to iron-56-particle radiation. Radiat Res. 2000. 154:28–33.
Article19. Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005. 78:3–7.
Article20. Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect. 1998. 106:Suppl 1. 277–283.
Article21. Feinendegen LE, Loken MK, Booz J, Muhlensiepen H, Sondhaus CA, Bond VP. Cellular mechanisms of protection and repair induced by radiation exposure and their consequences for cell system responses. Stem Cells. 1995. 13:Suppl 1. 7–20.22. Lee KS, Lim BV, Jang MH, Shin MC, Lee TH, Kim YP, Shin HS, Cho SY, Kim H, Shin MS, Kim EH, Kim CJ. Hypothermia inhibits cell proliferation and nitric oxide synthase expression in rats. Neurosci Lett. 2002. 329:53–56.
Article23. Revsin Y, Saravia F, Roig P, Lima A, de Kloet ER, Homo-Delarche F, De Nicola AF. Neuronal and astroglial alterations in the hippocampus of a mouse model for type 1 diabetes. Brain Res. 2005. 1038:22–31.
Article24. Shadley JD, Wiencke JK. Induction of the adaptive response by X-rays is dependent on radiation intensity. Int J Radiat Biol. 1989. 56:107–118.
Article25. Feinendegen LE, Bond VP, Sondhaus CA, Muehlensiepen H. Radiation effects induced by low doses in complex tissue and their relation to cellular adaptive responses. Mutat Res. 1996. 358:199–205.
Article26. Piotrowski P, Wierzbicka K, Smialek M. Neuronal death in the rat hippocampus in experimental diabetes and cerebral ischaemia treated with antioxidants. Folia Neuropathol. 2001. 39:147–154.27. Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002. 946:221–231.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Low Dose Radiation on the Neuronal Cell Proliferation in Diabetic Rats
- Adaptive Responses Induced by Low Dose Radiation in Dentate Gyrus of Rats
- Effects of hypothyroidism on cell proliferation and neuroblasts in the hippocampal dentate gyrus in a rat model of type 2 diabetes
- Cyanidin-3-glucoside inhibits amyloid β₂₅₋₃₅-induced neuronal cell death in cultured rat hippocampal neurons
- Protective Effect of Shenqi-wan on Traumatic Brain Injury-induced Delayed Apoptosis in Rat Hippocampal Dentate Gyrus