J Korean Med Sci.
2006 Dec;21(6):1103-1107. 10.3346/jkms.2006.21.6.1103.
Adaptive Responses Induced by Low Dose Radiation in Dentate Gyrus of Rats
- Affiliations
-
- 1Department of Radiation Oncology, Kyung Hee University Hospital, 1 Hoiki-dong, Dongdaemun-gu, Seoul, Korea. kangjino@khmc.or.kr
- 2Department of Physiology, School of Medicine, Kyung Hee University, Seoul, Korea.
- KMID: 1713127
- DOI: http://doi.org/10.3346/jkms.2006.21.6.1103
Abstract
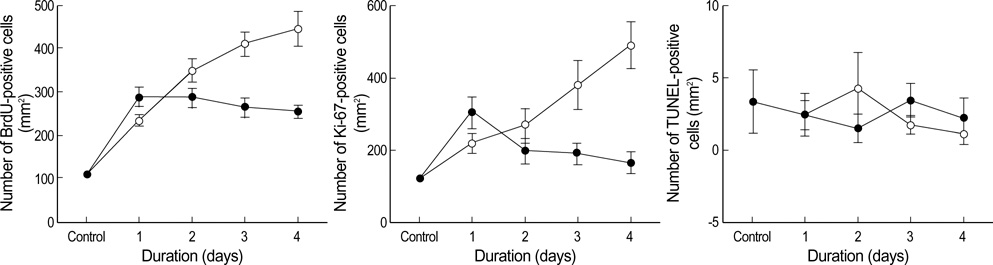
- The purpose of this study is to investigate the mechanism of alternative responses to low dose irradiation for neuronal cell proliferation in the dentate gyrus of rats. To determine the effect of a single exposure to radiation, rats were irradiated with a single dose of 0.1, 1, 10 or 20 Gy. To determine the effect of the cumulative dose, the animals were irradiated daily with 0.01 Gy or 0.1 Gy from 1 to 4 days. The neuronal cell proliferation was evaluated using immunohistochemistry for 5-bromo-2'-deoxyuridine (BrdU), Ki-67 and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining. Four consecutive daily irradiations with a 0.01 Gy/fraction increased the number of BrdU-positive and Ki-67-positive cells in a dose dependent manner, but this did not affect the number of TUNEL-positive cells. However, there was not a dose dependent relationship for the 0.1 Gy/fraction irradiation with the number of BrdU, Ki-67 and TUNEL positive cells. Our data support the explanation that the adaptive response, induced by low-dose radiation, in the hippocampus of rats is more likely a reflection of the perturbations of cell cycle progression.
MeSH Terms
-
Rats, Sprague-Dawley
Rats
Radiation Dosage
Neurons/*cytology/*radiation effects
Neuronal Plasticity/*radiation effects
Male
Dose-Response Relationship, Radiation
Dentate Gyrus/*cytology/*radiation effects
Cell Survival/radiation effects
Cell Proliferation/*drug effects
Animals
Adaptation, Physiological/radiation effects
Figure
Reference
-
1. Ferrer I, Borras D. Effects of X-irradiation on glial cells in the developing rat brain. Int J Radiat Biol. 1994. 66:181–187.
Article2. Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003. 63:4021–4027.3. Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000. 99:33–41.
Article4. Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003. 16:129–134.
Article5. Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005. 78:3–7.
Article6. Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984. 223:594–597.
Article7. Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect. 1998. 106:Suppl 1. 277–283.
Article8. Feinendegen LE, Loken MK, Booz J, Muhlensiepen H, Sondhaus CA, Bond VP. Cellular mechanisms of protection and repair induced by radiation exposure and their consequences for cell system responses. Stem Cells. 1995. 13:Suppl 1. 7–20.9. Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998. 18:7768–7778.
Article10. Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001. 98:4710–4715.
Article11. Kang JO, Kim SK, Hong SE, Lee TH, Kim CJ. Low dose radiation overcomes diabetes-induced suppression of hippocampal neuronal cell proliferation in rats. J Korean Med Sci. 2006. 21:500–505.
Article12. Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002. 115:97–105.
Article13. Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998. 4:1313–1317.
Article14. Peissner W, Kocher M, Treuer H, Gillardon F. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res. 1999. 71:61–68.15. Hodges H, Katzung N, Sowinski P, Hopewell JW, Wilkinson JH, Bywaters T, Rezvani M. Late behavioural and neuropathological effects of local brain irradiation in the rat. Behav Brain Res. 1998. 91:99–114.
Article16. Sienkiewicz ZJ, Haylock RG, Saunders RD. Prenatal irradiation and spatial memory in mice: investigation of dose-response relationship. Int J Radiat Biol. 1994. 65:611–618.
Article17. Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002. 8:955–962.
Article18. Shadley JD, Wiencke JK. Induction of the adaptive response by X-rays is dependent on radiation intensity. Int J Radiat Biol. 1989. 56:107–118.
Article19. Feinendegen LE, Bond VP, Sondhaus CA, Muehlensiepen H. Radiation effects induced by low doses in complex tissue and their relation to cellular adaptive responses. Mutat Res. 1996. 358:199–205.
Article20. Liu SZ, Zhang YC, Mu Y, Su X, Liu JX. Thymocyte apoptosis in response to low-dose radiation. Mutat Res. 1996. 358:185–191.
Article21. Shaposhnikova VV, Korystov YN. Thymocyte proliferation and apoptosis induced by ionizing radiation. Scanning Microsc. 1995. 9:1203–1206.22. Ikushima T, Aritomi H, Morisita J. Radioadaptive response: efficient repair of radiation-induced DNA damage in adapted cells. Mutat Res. 1996. 358:193–198.
Article23. Johansson L. Hormesis, an update of the present position. Eur J Nucl Med Mol Imaging. 2003. 30:921–933.
Article24. Cramers P, Atanasova P, Vrolijk H, Darroudi F, van Zeeland AA, Huiskamp R, Mullenders LH, Kleinjans JC. Pre-exposure to low doses: modulation of X-ray-induced dna damage and repair? Radiat Res. 2005. 164(4 Pt 1):383–390.
Article25. Braun N, Papadopoulos T, Muller-Hermelink HK. Cell cycle dependent distribution of the proliferation-associated Ki-67 antigen in human embryonic lung cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988. 56:25–33.
Article26. Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999. 2:260–265.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Low Dose Radiation Overcomes Diabetes-induced Suppression of Hippocampal Neuronal Cell Proliferation in Rats
- The Effect of Low Dose Radiation on the Neuronal Cell Proliferation in Diabetic Rats
- Expression Changes of c-Fos Protein of Rat Brain Following Pentylenetetrazol-induced Seizures
- Low-Dose Radiation-Induced Effects on Cognitive Function
- Adaptive response to ionizing radiation induced by low dose of gamma ray in human hepatoma cell lines