J Korean Med Sci.
2006 Jun;21(3):379-384. 10.3346/jkms.2006.21.3.379.
Colocalization of Interferon Regulatory Factor 7 (IRF7) with Latent Membrane Protein 1 (LMP1) of Epstein-Barr Virus
- Affiliations
-
- 1Department of Applied Microbiology, College of Natural Resources, Yeungnam University, Daegu, Korea.
- 2Department of Microbiology, College of Medicine, Yeungnam University, Daegu, Korea. hspark@med.yu.ac.kr
- KMID: 1778415
- DOI: http://doi.org/10.3346/jkms.2006.21.3.379
Abstract
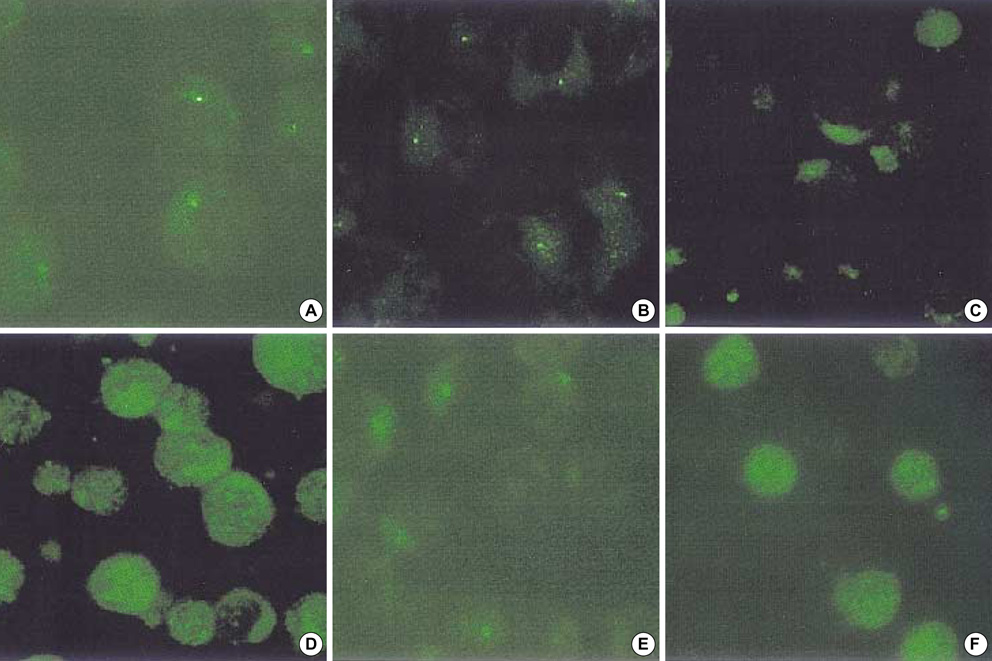
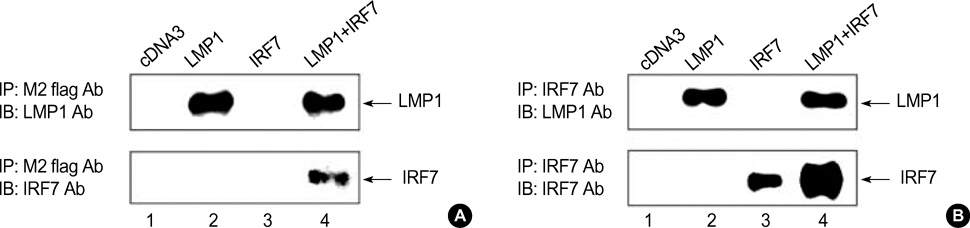
- Interferon regulatory factor 7 (IRF7) is one of the transcriptional factors for the activation of type I Interferon (IFN) genes. It is known that IRF7 and the latent membrane protein 1 (LMP1) of Epstein-Barr virus (EBV) are highly expressed in EBV type III latency cells, and LMP1 induces mRNA expression of IRF7. In this study, the expression pattern of endogenous IRF7 was observed in several B cell lines with or without EBV infection by immunofluorescence staining. IRF7 was localized in the cytoplasm of EBV-negative B cells and EBV type I latency B cell lines. However, IRF7 was located both in the cytoplasm and nucleus of EBV type III latency cell lines. In the Jijoye cell (type III latency cell), IRF7 was colocalized with LMP1 in the cytoplasm in a capping configuration, and their interaction was confirmed by co-immunoprecipitation of LMP1 and IRF7. This colocalization was confirmed by co-transfection of IRF7 and LMP1 plasmids in EBV-negative B cells. These results suggest that the IRF7 and LMP1 interact with each other, and this may relate to the mechanism whereby LMP1 exerts functional effects in B-lymphocytes.
Keyword
MeSH Terms
-
Viral Matrix Proteins/*biosynthesis/metabolism
Trans-Activation (Genetics)
Signal Transduction
RNA, Messenger/metabolism
Plasmids/metabolism
Microscopy, Fluorescence
Interferon Regulatory Factor-7/*biosynthesis
Immunoprecipitation
Humans
Herpesvirus 4, Human/metabolism
*Gene Expression Regulation
Cytoplasm/metabolism
Cell Line, Tumor
B-Lymphocytes/metabolism/virology
Figure
Reference
-
1. Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993. 90:9150–9154.
Article2. Hennessy S, Fennewald S, Hummel M, Cole T, Kieff E. A membrane protein encoded by Epstein-Barr virus in latent growth-transforming infection. Proc Natl Acad Sci USA. 1984. 81:7207–7211.
Article3. Liebowitz D, Wang D, Kieff E. Orientation and patching of the latent infection membrane protein encoded by Epstein-Barr virus. J Virol. 1986. 58:233–237.
Article4. Longnecker R, Kieff E. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990. 64:2319–2326.
Article5. Longnecker R, Druker B, Roberts TM, Kieff E. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J Virol. 1991. 65:3681–3692.
Article6. Li HP, Chang YS. Epstein-Barr virus latent membrane protein 1: structure and functions. J Biomed Sci. 2003. 10:490–504.
Article7. Nguyen H, Hiscott J, Pitha PM. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 1997. 8:293–312.
Article8. Kimura T, Nakayama K, Penninger J, Kitagawa M, Harada H, Matsuyama T, Tanaka N, Kamijo R, Vilcek J, Mak TW, Taniguchi T. Involvement of the IRF-1 transcription factor in antiviral responses to interferons. Science. 1994. 264:1921–1924.
Article9. Au WC, Moore PA, Lowther W, Juang YT, Pitha PM. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc Natl Acad Sci USA. 1995. 92:11657–11661.
Article10. Harada H, Kitagawa M, Tanaka N, Yamamoto H, Harada K, Ishihara M, Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993. 259:971–974.
Article11. Tanaka N, Ishihara M, Kitagawa M, Harada H, Kimura T, Matsuyama T, Lamphier MS, Aizawa S, Mak TW, Taniguchi T. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell. 1994. 77:829–839.
Article12. Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000. 13:539–548.13. Zhang L, Pagano JS. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997. 17:5748–5757.
Article14. Au WC, Moore PA, LaFleur DW, Tombal B, Pitha PM. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J Biol Chem. 1998. 273:29210–29217.
Article15. Lin R, Mamane Y, Hiscott J. Multiple regulatory domains control IRF-7 activity in response to virus infection. J Biol Chem. 2000. 275:34320–34327.
Article16. Sharma S, tenOver BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003. 300:1148–1151.
Article17. Zhang L, Pagano JS. Interferon regulatory factor 7 is induced by Epstein-Barr virus latent membrane protein 1. J Virol. 2000. 74:1061–1068.
Article18. Zhang L, Wu L, Hong K, Pagano JS. Intracellular signaling molecules activated by Epstein-Barr virus for induction of interferon regulatory factor 7. J Virol. 2001. 75:12393–12401.
Article19. Ning S, Hahn AM, Huye LE, Pagano JS. Interferon regulatory factor 7 regulates expression of Epstein-Barr virus latent membrane protein 1: a regulatory circuit. J Virol. 2003. 77:9359–9368.
Article20. Ning S, Huye LE, Pagano JS. Interferon regulatory factor 5 represses expression of the Epstein-Barr virus oncoprotein LMP1: braking of the IRF7/LMP1 regulatory circuit. J Virol. 2005. 79:11671–11676.
Article21. Ben-Bassat H, Goldblum N, Mitrani S, Goldblum T, Yoffey JM, Cohen MM, Bentwich Z, Ramot B, Klein E, Klein G. Establishment in continuous culture of a new type of lymphocyte from a "Burkitt like" malignant lymphoma (line D.G.-75). Int J Cancer. 1977. 19:27–33.22. Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991. 5:147–156.
Article23. Ragona G, Ernberg I, Klein G. Induction and biological characterization of the Epstein-Barr virus (EBV) carried by the Jijoye lymphoma line. Virology. 1980. 101:553–557.
Article24. Nonkwelo C, Skinner J, Bell A, Rickinson A, Sample J. Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early-passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. J Virol. 1996. 70:623–627.
Article25. Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998. 17:6660–6669.
Article26. Au WC, Yeow WS, Pitha PM. Analysis of functional domains of interferon regulatory factor 7 and its association with IRF-3. Virology. 2001. 280:273–282.
Article27. Marie I, Smith E, Prakash A, Levy DE. Phosphorylation-induced dimerization of interferon regulatory factor 7 unmasks DNA binding and a bipartite transactivation domain. Mol Cell Biol. 2000. 20:8803–8814.28. Tjian R, Maniatis T. Transcriptional activation: a complex puzzle with few easy pieces. Cell. 1994. 77:5–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Synergistic Effect of LMP1 and IRF7 on the Expression of TAP1 in DG 75 Cell Line
- ASK1 is Involved in EBV LMP1-induced NF-kappaB Activation
- Genetic Analysis of Epstein-Barr Virus Latent Membrane Protein 1 and Immunohistochemical Expression of Transforming Growth Factor (TGF)-beta1, TGF-betaRII, p21, p16, E2F1, Thymidylate Synthase, and NF-kappaB in Epstein-Barr Virus Encoded RNA-positive Gastric Adenocarcinoma
- Epstein-Barr virus latent genes
- Detection of Epstein-barr virus by PCR and expression of LMP1, p53, CD44 in gastric cancer