Yonsei Med J.
2012 Jul;53(4):834-841. 10.3349/ymj.2012.53.4.834.
Capsaicin-Induced Apoptosis of FaDu Human Pharyngeal Squamous Carcinoma Cells
- Affiliations
-
- 1Department of Pharmacology and Dental Therapeutics, School of Dentistry, Chosun University, Gwangju, Korea. hoon_yoo@chosun.ac.kr
- 2Department of Food Science and Nutrition, University of Ulsan, Ulsan, Korea.
- KMID: 1716886
- DOI: http://doi.org/10.3349/ymj.2012.53.4.834
Abstract
- PURPOSE
To investigate the anti-tumor effect of capsaicin on human pharyngeal squamous carcinoma cells (FaDu).
MATERIALS AND METHODS
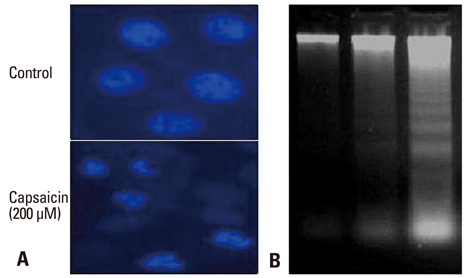
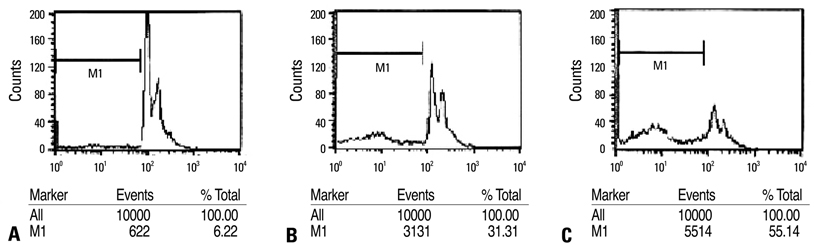
The expression of apoptosis/cell cycle-related proteins (or genes) was examined by reverse transcriptase-polymerase chain reaction, western blotting and ELISA methods, while the apoptotic cell population, cell morphology and DNA fragmentation levels were assessed using flow cytometry, fluorescence microscopy and agarose gel electrophoresis.
RESULTS
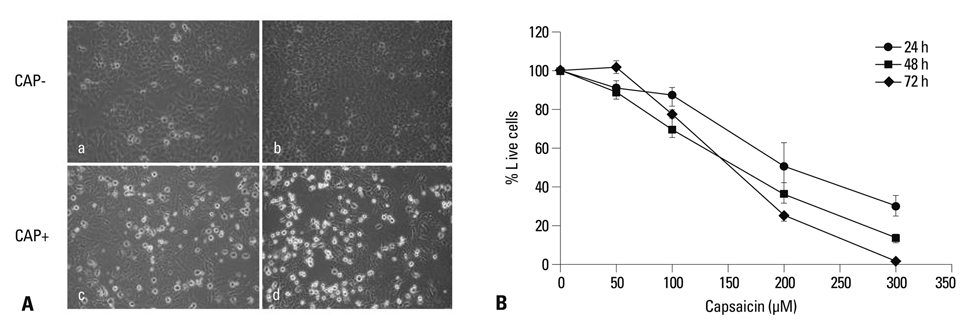
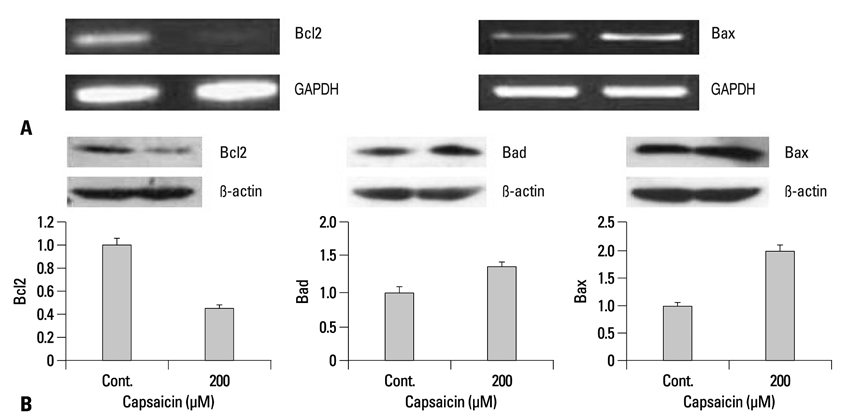
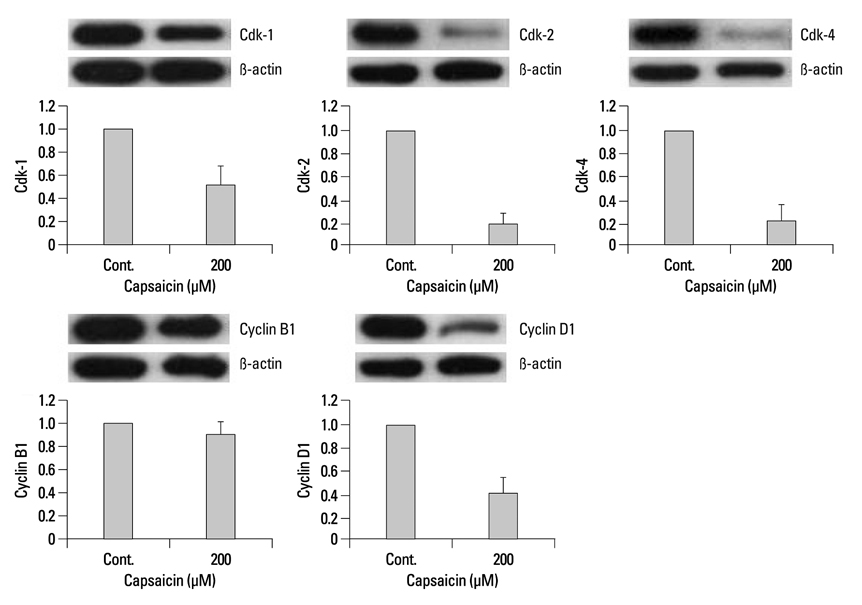
Capsaicin was found to inhibit the growth and proliferation of FaDu cells in a dose- and time-dependent manner. Apoptotic cell death was confirmed by observing increases in nuclear condensation, nuclear DNA fragmentation and sub-G1 DNA content. The observed increase in cytosolic cytochrome c, activation of caspase 3 and PARP (p85) levels following capsaicin treatment indicated that the apoptotic response was mitochondrial pathway-dependent. Gene/protein expression analysis of Bcl-2, Bad and Bax further revealed decreased anti-apoptotic Bcl-2 protein and increased pro-apoptotic Bad/Bax expression. Furthermore, capsaicin suppressed the cell cycle progression at the G1/S phase in FaDu cells by decreasing the expression of the regulators of cyclin B1 and D1, as well as cyclin-dependent protein kinases cdk-1, cdk-2 and cdk-4.
CONCLUSION
Our current data show that capsaicin induces apoptosis in FaDu cells and this response is associated with mitochondrial pathways, possibly by mediating cell cycle arrest at G1/S.
MeSH Terms
-
Apoptosis/drug effects
Blotting, Western
Capsaicin/*pharmacology
Carcinoma, Squamous Cell/*metabolism
Cell Cycle/drug effects
Cell Line, Tumor
Cell Proliferation/drug effects
Enzyme-Linked Immunosorbent Assay
Flow Cytometry
Humans
Microscopy, Fluorescence
Pharyngeal Neoplasms/*metabolism
Proto-Oncogene Proteins c-bcl-2/genetics/metabolism
Reverse Transcriptase Polymerase Chain Reaction
bcl-2-Associated X Protein/genetics/metabolism
bcl-Associated Death Protein/genetics/metabolism
Figure
Reference
-
1. Chen AY, Myers JN. Cancer of the oral cavity. Curr Probl Surg. 2000. 37:633–731.
Article2. Figuero Ruiz E, Carretero Peláez MA, Cerero Lapiedra R, Esparza Gómez G, Moreno López LA. Effects of the consumption of alcohol in the oral cavity: relationship with oral cancer. Med Oral. 2004. 9:14–23.3. Pintos J, Black MJ, Sadeghi N, Ghadirian P, Zeitouni AG, Viscidi RP, et al. Human papillomavirus infection and oral cancer: a case-control study in Montreal, Canada. Oral Oncol. 2008. 44:242–250.
Article4. Day TA, Davis BK, Gillespie MB, Joe JK, Kibbey M, Martin-Harris B, et al. Oral cancer treatment. Curr Treat Options Oncol. 2003. 4:27–41.
Article5. Yang KM, Pyo JO, Kim GY, Yu R, Han IS, Ju SA, et al. Capsaicin induces apoptosis by generating reactive oxygen species and disrupting mitochondrial transmembrane potential in human colon cancer cell lines. Cell Mol Biol Lett. 2009. 14:497–510.
Article6. Oyagbemi AA, Saba AB, Azeez OI. Capsaicin: a novel chemopreventive molecule and its underlying molecular mechanisms of action. Indian J Cancer. 2010. 47:53–58.
Article7. Huang SP, Chen JC, Wu CC, Chen CT, Tang NY, Ho YT, et al. Capsaicin-induced apoptosis in human hepatoma HepG2 cells. Anticancer Res. 2009. 29:165–174.8. Kim JD, Kim JM, Pyo JO, Kim SY, Kim BS, Yu R, et al. Capsaicin can alter the expression of tumor forming-related genes which might be followed by induction of apoptosis of a Korean stomach cancer cell line, SNU-1. Cancer Lett. 1997. 120:235–241.
Article9. Yoshitani SI, Tanaka T, Kohno H, Takashima S. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by dietary capsaicin and rotenone. Int J Oncol. 2001. 19:929–939.
Article10. Morré DJ, Chueh PJ, Morré DM. Capsaicin inhibits preferentially the NADH oxidase and growth of transformed cells in culture. Proc Natl Acad Sci U S A. 1995. 92:1831–1835.
Article11. Jung MY, Kang HJ, Moon A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001. 165:139–145.
Article12. Lee YS, Nam DH, Kim JA. Induction of apoptosis by capsaicin in A172 human glioblastoma cells. Cancer Lett. 2000. 161:121–130.
Article13. Lee JM, Moehlenkamp JD, Hanson JM, Johnson JA. Nrf2-dependent activation of the antioxidant responsive element by tert-butylhydroquinone is independent of oxidative stress in IMR-32 human neuroblastoma cells. Biochem Biophys Res Commun. 2001. 280:286–292.
Article14. Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999. 51:159–212.15. Cho YS, Park SY, Lee CK, Lee EY, Shin JH, Yoo B, et al. Enhanced cough response to hyperpnea with cold air challenge in chronic cough patients showing increased cough sensitivity to inhaled capsaicin. Allergy. 2003. 58:486–491.
Article16. Amantini C, Mosca M, Nabissi M, Lucciarini R, Caprodossi S, Arcella A, et al. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007. 102:977–990.
Article17. Kim MY, Trudel LJ, Wogan GN. Apoptosis induced by capsaicin and resveratrol in colon carcinoma cells requires nitric oxide production and caspase activation. Anticancer Res. 2009. 29:3733–3740.18. Kim CS, Park WH, Park JY, Kang JH, Kim MO, Kawada T, et al. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor gamma in HT-29 human colon cancer cells. J Med Food. 2004. 7:267–273.
Article19. Ip SW, Lan SH, Huang AC, Yang JS, Chen YY, Huang HY, et al. Capsaicin induces apoptosis in SCC-4 human tongue cancer cells through mitochondria-dependent and -independent pathways. Environ Toxicol. 2010. [Epub ahead of print].
Article20. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998. 281:1309–1312.
Article21. Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000. 10:369–377.
Article22. Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996. 86:147–157.
Article23. Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003. 39:615–647.
Article24. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999. 397:441–446.
Article25. Gil YG, Kang MK. Capsaicin induces apoptosis and terminal differentiation in human glioma A172 cells. Life Sci. 2008. 82:997–1003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to "Capsaicin-Induced Apoptosis of FaDu Human Pharyngeal Squamous Carcinoma Cells" by Le TD, et al. (Yonsei Med J 2012;53:834-41.)
- Effects of cimifugin on cell growth inhibition and cell apoptosis induction in fadu human pharyngeal squamous cell carcinoma
- Inhibition of cell growth and induction of apoptosis by bilobalide in FaDu human pharyngeal squamous cell carcinoma
- Apoptotic effects of hyperoside on FaDu human pharyngeal carcinoma cells
- Apoptotic effect of betulinic acid in FaDu human head and neck squamous cell carcinomas