J Korean Med Sci.
2005 Aug;20(4):628-635. 10.3346/jkms.2005.20.4.628.
Expression and Regulation of Latent TGF-beta Binding Protein-1 Transcripts and Their Splice Variants in Human Glomerular Endothelial Cells
- Affiliations
-
- 1Hyonam Kidney Laboratory, Soon Chun Hyang University, Korea. hblee@hkl.ac.kr
- 2Department of Internal Medicine, Soon Chun Hyang University College of Medicine, Seoul, Korea.
- KMID: 1712745
- DOI: http://doi.org/10.3346/jkms.2005.20.4.628
Abstract
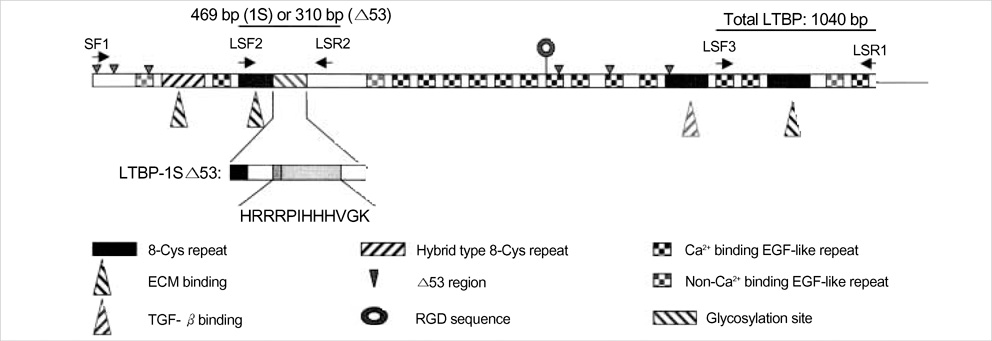
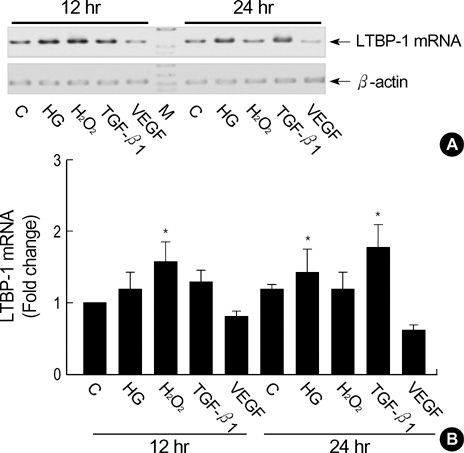
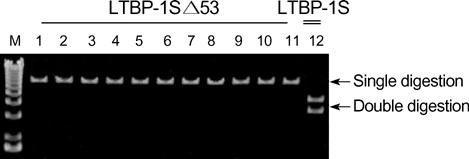
- Latent transforming growth factor (TGF)-beta-binding protein (LTBP) is required for the assembly, secretion, matrix association, and activation of latent TGF-beta complex. To elucidate the cell specific expression of the genes of LTBP-1 and their splice variants and the factors that regulate the gene expression, we cultured primary human glomerular endothelial cells (HGEC) under different conditions. Basal expression of LTBP-1 mRNA was suppressed in HGEC compared to WI-38 human embryonic lung fibroblasts. High glucose, H2O2, and TGF-beta1 upregulated and vascular endothelial growth factor (VEGF) further downregulated LTBP-1 mRNA in HGEC. RT-PCR with a primer set for LTBP-1S produced many clones but no clone was gained with a primer set for LTBP-1L. Of 12 clones selected randomly, Sca I mapping and DNA sequencing revealed that only one was LTBP-1S and all the others were LTBP-1S delta 53. TGF-beta1, but not high glucose, H2O2 or VEGF, tended to increase LTBP-1S delta 53 mRNA. In conclusion, HGEC express LTBP-1 mRNA which is suppressed at basal state but upregulated by high glucose, H2O2, and TGF-beta1 and downregulated by VEGF. Major splice variant of LTBP-1 in HGEC was LTBP-1S delta 53. Modification of LTBP-1S delta 53 gene in HGEC may abrogate fibrotic action of TGF-beta1 but this requires confirmation.
Keyword
MeSH Terms
-
*Alternative Splicing
Amino Acid Sequence
Cell Line
Cells, Cultured
Cloning, Molecular
Comparative Study
Endothelial Cells/drug effects/*metabolism
*Gene Expression Regulation
Glucose/pharmacology
Humans
Hydrogen Peroxide/pharmacology
Intracellular Signaling Peptides and Proteins/*genetics
Kidney Glomerulus/cytology
Protein Isoforms/genetics
RNA, Messenger/genetics/metabolism
Research Support, Non-U.S. Gov't
Reverse Transcriptase Polymerase Chain Reaction
*Transcription, Genetic
Transfection
Transforming Growth Factor beta/pharmacology
Vascular Endothelial Growth Factor A/pharmacology
Figure
Reference
-
1. Miyazono K, Hellman U, Wernstedt C, Heldin CH. Latent high molecular weight complex of transforming growth factor-β1. Purification from human platelets and structural characterization. J Biol Chem. 1988. 263:6407–6415.2. Olofsson A, Miyazono K, Kanzaki T, Colosetti P, Engstrom U, Heldin CH. Transforming growth factor-β1, -β2, and -β3 secreted by a human glioblastoma cell line. Identification of small and different forms of large latent complexes. J Biol Chem. 1992. 267:19482–19488.3. Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-β1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995. 270:4689–4696.
Article4. Wakefield LM, Smith DM, Flanders KC, Sporn MB. Latent transforming growth factor-β from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988. 263:7646–7654.5. Kanzaki T, Olofsson A, Moren A, Wernstedt C, Hellman U, Miyazono K, Claesson-Welsh L, Heldin CH. TGF-β1 binding protein: a component of the large latent complex of TGF-β1 with multiple repeat sequences. Cell. 1990. 61:1051–1061.
Article6. Tsuji T, Okada F, Yamaguchi K, Nakamura T. Molecular cloning of the large subunit of transforming growth factor type β masking protein and expression of the mRNA in various rat tissues. Proc Natl Acad Sci USA. 1990. 87:8835–8839.7. Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor-β1 precursor by human furin convertase. J Biol Chem. 1995. 270:618–624.8. Gentry LE, Lioubin MN, Purchio AF, Marquardt H. Molecular events in the processing of recombinant type I pre-pro-transforming growth factor β to the mature polypeptide. Mol Cell Biol. 1988. 8:4162–4168.9. Saharinen J, Taipale J, Keski-Oja J. Association of the small latent transforming growth factor-β with an eight cysteine repeat of its binding protein LTBP-1. EMBO J. 1996. 15:245–253.10. Gleizes PE, Beavis RC, Mazzieri R, Shen B, Rifkin DB. Identification and characterization of an eight-cysteine repeat of the latent transforming growth factor-β binding protein-1 that mediates bonding to the latent transforming growth factor-β1. J Biol Chem. 1996. 271:29891–29896.
Article11. Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role of the latent TGF-β1-binding protein in the assembly and secretion of TGF-β1. EMBO J. 1991. 10:1091–1101.12. Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-β associates to fibroblast extracellular matrix via latent TGF-β1 binding protein. J Cell Biol. 1994. 124:171–181.13. Flaumenhaft R, Abe M, Sato Y, Miyazono K, Harpel J, Heldin CH, Rifkin DB. Role of the latent TGF-β binding protein in the activation of latent TGF-β by co-cultures of endothelial and smooth muscle cells. J Cell Biol. 1993. 120:995–1002.14. Dallas SL, Miyazono K, Skerry TM, Mundy GR, Bonewald LF. Dual role for the latent transforming growth factor-β binding protein in storage of latent TGF-β in the extracellular matrix and as a structural matrix protein. J Cell Biol. 1995. 131:539–549.15. Moren A, Olofsson A, Stenman G, Sahlin P, Kanzaki T, Clasesson-Welsh L, ten Dijke P, Miyazono K, Heldin CH. Identification and characterization of LTBP-2, a novel latent transforming growth factor-β-binding protein. J Biol Chem. 1994. 269:32469–32478.16. Olofsson A, Ichijo H, Moren A, ten Dijke P, Miyazono K, Heldin CH. Efficient association of an amino-terminally extended form of human latent transforming growth factor-β binding protein with the extracellular matrix. J Biol Chem. 1995. 270:31294–31297.
Article17. Koski C, Saharinen J, Keski-Oja J. Independent promoters regulate the expression of two amino terminally distinct forms of latent transforming growth factor-β binding protein-1 (LTBP-1) in a cell type-specific manner. J Biol Chem. 1999. 274:32619–32630.
Article18. Mangasser-Stephan K, Gartung C, Lahme B, Gressner AM. Expression of isoforms and splice variants of the latent transforming growth factor beta binding protein (LTBP) in cultured human liver myofibroblasts. Liver. 2001. 21:105–113.19. Weikkolainen K, Keski-Oja J, Koli K. Expression of latent TGF-beta binding protein LTBP-1 is hormonally regulated in normal and transformed human lung fibroblasts. Growth Factors. 2003. 21:51–60.20. Wada T, Hamakawa S, Hori Y, Kaname S, Shimizu S, Kurokawa K, Katoh T. Immunohistochemical localization of latent transforming growth factor-β binding protein in IgA nephropathy. Kidney Int. 1997. 52:S182–S184.21. Kinnman N, Andersson U, Hultcrantz R. In situ expression of transforming growth factor-beta1-3, latent transforming growth factor-beta binding protein and tumor necrosis factor-alpha in liver tissue from patients with chronic hepatitis C. Scand J Gastroenterol. 2000. 35:1294–1300.22. Khalil N, Parekh TV, O'Connor R, Antman N, Kepron W, Yehaulaeshet T, Xu YD, Gold LI. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 2001. 56:907–915.23. Saika S, Miyamoto T, Tanaka T, Ishida I, Ohnishi Y, Ooshima A. Latent TGF-beta binding protein-1 and fibrillin-1 in human capsular opacification and in cultured lens epithelial cells. Br J Ophthalmol. 2001. 85:1362–1366.
Article24. Higashi T, Sasagawa T, Inoue M, Oka R, Shuangying L, Saijoh K. Overexpression of latent transforming growth factor-beta 1 (TGF-beta 1) binding protein 1 (LTBP-1) in association with TGF-beta 1 in ovarian carcinoma. Jpn J Cancer Res. 2001. 92:506–515.25. Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994. 331:1286–1292.
Article26. Lee LK, Meyer TW, Pollock AS, Lovett DH. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest. 1995. 96:953–964.
Article27. Lee HB, Cha MK, Song KI, Kim JH, Lee EA, Kim SI, Kim J, Yoo MH. Pathogenic role of advanced glycosylation end products in diabetic nephropathy. Kidney Int. 1997. 60:S60–S65.28. Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993. 90:1814–1818.
Article29. Oh JH, Ha H, Yu MR, Lee HB. Sequential effects of high glucose on mesangial cell transforming growth factor-β1 and fibronectin synthesis. Kidney Int. 1998. 54:1872–1878.
Article30. van Det NF, Verhagen NA, Tamsma JT, Berden JH, Bruijn JA, Daha MR, van der Woude FJ. Regulation of glomerular epithelial cell production of fibronectin and transforming growth factor-β by high glucose, not by angiotensin II. Diabetes. 1997. 46:834–840.
Article31. Rocco M, Chen Y, Goldfarb S, Ziyadeh FN. Elevated glucose stimulates TGF-β gene expression and bioactivity in proximal tubule. Kidney Int. 1992. 41:107–114.
Article32. Green DF, Hwang KH, Ryan US, Bourgoignie JJ. Culture of endothelial cells from baboon and human glomeruli. Kidney Int. 1992. 41:1506–1516.
Article33. Park S, Ahn H, Kim SW, Lee JD, Park JS. Culture of human glomerular endothelial cells. Korean J Nephrol. 1997. 16:221–229.34. Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003. 14:S241–S245.
Article35. Roth-Eichhorn S, Heitmann B, Flemming P, Kubicka S, Trautwein C. Evidence for the decreased expression of the latent TGF-beta binding protein and its splice form in human liver tumors. Scand J Gastroenterol. 2001. 36:1204–1210.36. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999. 13:9–22.
Article37. Kobayashi T, Liu X, Wen FQ, Fang Q, Abe S, Wang XQ, Hashimoto M, Shen L, Kawasaki S, Kim HJ, Kohyama T, Rennard Si. Smad3 mediates TGF-beta1 induction of VEGF production in lung fibroblasts. Biochem Biophys Res Commun. 2005. 327:393–398.38. Yamaguchi K, Nishimura Y, Shigematsu S, Takeuchi Y, Nakamura J, Aizawa T, Hashizume K. Vascular endothelial cell growth factor attenuates actions of transforming growth factor-beta in human endothelial cells. J Biol Chem. 2004. 279:55104–55108.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transforming growth factor-beta and the glomerular filtration barrier
- The Effect of Dexamethasone and Transforming Growth Factor-beta1 on the Cytokine Induced Expression of VCAM-1 in Glomerular Endothelial Cells
- Transforming growth factor beta 1 expression in gastric carcinoma
- Expression of TGF-beta1 and TGF-betatype II Receptor in Chemically Induced Hepatocarcinogenesis of the Rat
- Differential Expression of TGF-beta Isoforms During Differentiation of HaCaT Human Keratinocyte Cells: Implication for the Separate Role in Epidermal Differentiation