J Korean Med Sci.
2005 Aug;20(4):555-561. 10.3346/jkms.2005.20.4.555.
IL-1beta Acts in Synergy with Endogenous IL-1beta in A375-S2 Human Melanoma Cell Apoptosis Through Mitochondrial Pathway
- Affiliations
-
- 1China-Japan Research Institute of Medical and Pharmaceutical Sciences, Shenyang Pharmaceutical University, China. ikejimat@vip.sina.com
- 2Department of Pharmacology, Shenyang Pharmaceutical University, Shenyang, China.
- 3Department of Clinical and Biomedical Sciences, Showa Pharmaceutical University, Tokyo, Japan.
- KMID: 1712732
- DOI: http://doi.org/10.3346/jkms.2005.20.4.555
Abstract
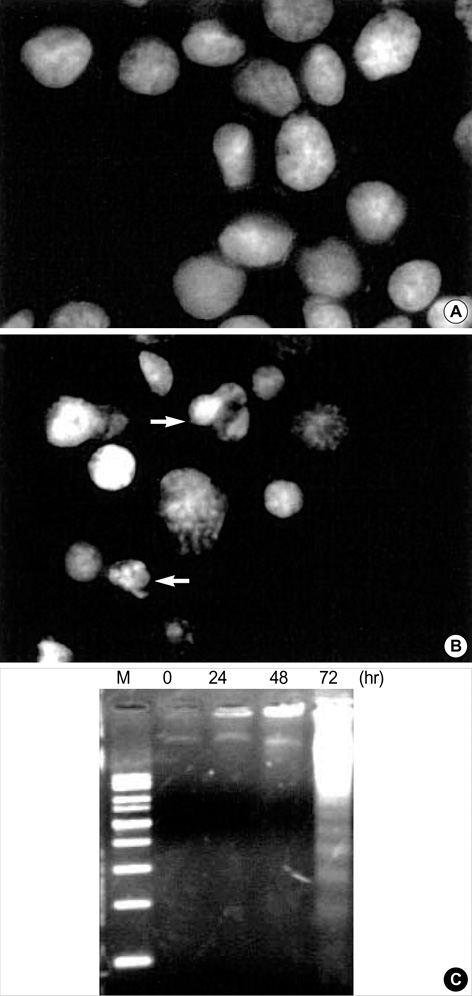
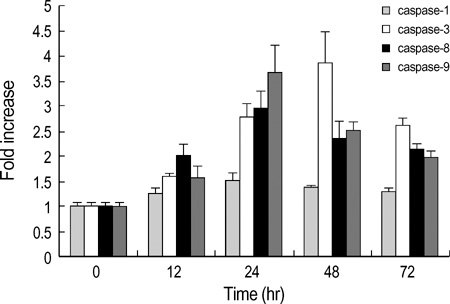
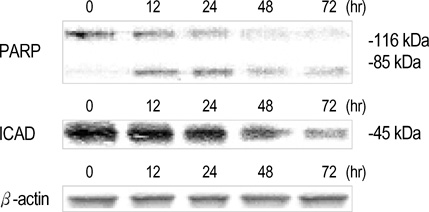
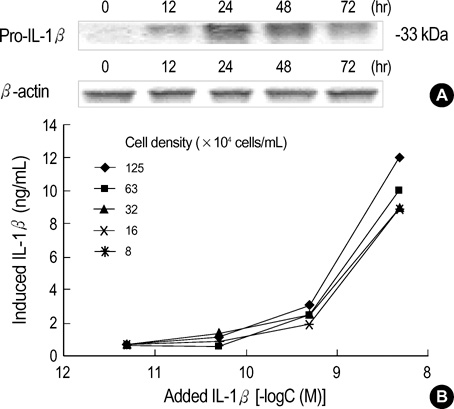
- Interleukin-1beta (IL-1beta) is a pivotal proinflammatory cytokine. To investigate the mechanism of IL-1beta-induced cell death in human malignant melanoma A375-S2 cells, MTT assay, photomicroscopical observation, DNA agarose gel electrophoresis, radioimmunoassay and Western blot analysis were carried out. IL-1beta did not only induce nuclear condensation and DNA fragmentation, but also increased degradation of two substrates of caspase-3, poly ADP-ribose polymerase (PARP) and inhibitor of caspase-activated DNase (ICAD). Simultaneously, release of precursor of IL-1beta (pro-IL-1beta) and endogenous IL-1beta production were involved in the apoptotic process. IL-1beta enhanced the ratio of Bax/Bcl-2 and Bax/Bcl-xL expression and up-regulated apoptosis inducing factor (AIF) expression, which required the activation of downstream caspases. These results suggest that IL-1beta induces endogenous IL-1beta production, enhances cleavage of caspase downstream substrates and promotes mitochondria mediated apoptosis in A375-S2 cells.
Keyword
MeSH Terms
-
Apoptosis/*drug effects
Blotting, Western
Caspase 1/metabolism
Caspases/metabolism
Cell Line, Tumor
Cell Proliferation/drug effects
Cell Survival/drug effects
Comparative Study
DNA Fragmentation/drug effects
Deoxyribonucleases/metabolism
Dose-Response Relationship, Drug
Enzyme Activation/drug effects
Humans
Interleukin-1/biosynthesis/*pharmacology/physiology
Interleukin-6/pharmacology
Lymphotoxin/pharmacology
Melanoma/metabolism/pathology/physiopathology
Mitochondria/*physiology
Poly(ADP-ribose) Polymerases/metabolism
Proto-Oncogene Proteins c-bcl-2/biosynthesis
Time Factors
Figure
Reference
-
1. Pascale RL. The Interleukin-1 family. Eur Cytokine Netw. 1998. 9:565–576.2. Dinarello CA. Biologiical basis for interleukin-1 in disease. Blood. 1996. 87:2095–2147.3. Burger D, Molnarfi N, Gruaz L, Dayer JM. Differential induction of IL-1beta and TNF by CD40 ligand or cellular contact with stimulated T cells depends on the maturation stage of human monocytes. J Immunol. 2004. 173:1292–1297.4. Freedman AS, Freeman G, Whitman J, Segil J, Daley J, Nadler LM. Pre-exposure of human B cells to recombinant IL-1 enhances subsequent proliferation. J Immunol. 1988. 141:3398–3404.5. Vesey DA, Cheung C, Cuttle L, Endre Z, Gobe G, Johnson DW. Interleukin-1beta stimulates human renal fibroblast proliferation and matrix protein production by means of a transforming growth factor-beta-dependent mechanism. J Lab Clin Med. 2002. 140:342–350.6. Maier JA, Voulalas P, Roeder D, Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1α antisense oligomer. Science. 1990. 249:1570–1574.7. Komeva EA, Shanin SN, Rybakina EG. The role of interleukin-1 in stress-induced changes in immune system function. Neurosci Behav Physiol. 2001. 31:431–437.8. Onozaki K, Tamatani T, Hashimoto T, Matsushima K. Growth inhibition and augmentation of mouse myeloid leukemic cell line differentiation by Interleukin-1. Cancer Res. 1987. 47:2397–2402.9. Lachman LB, Dinarello CA, Llansa ND, Fidler IJ. Natural and recombinant human interleukin 1-beta is cytotoxic for human melanoma cells. J Immunol. 1986. 136:3098–3102.10. Endo Y, Matsushima K, Onozaki K, Oppenheim JJ. Role of ornithine decarboxylase in the regulation of cell growth by IL-1 and tumor necrosis factor. J Immunol. 1988. 141:2342–2348.11. Yang D, Hayashi H, Takii T, Mizutani Y, Inukai Y, Onozaki K. Interleukin-1-induced growth inhibition of human melanoma cells. J Biol Chem. 1997. 272:3376–3383.
Article12. Morinaga Y, Suzuki H, Takatsuki F, Akiyama Y, Taniyama T, Matsushima K, Onozaki K. Contribution of IL-6 to the antiproliferative effect of IL-1 and tumor necrosis factor on tumor cell lines. J Immunol. 1989. 143:3538–3542.13. Murai T, Yukari N, Hideko M, Terada K. Altered regulation of cell cycle machinery involved in interleukin-1-induced G1 and G2 phase growth arrest of A375S2 human melanoma cells. J Biol Chem. 2001. 276:6797–6806.
Article14. Itoh S, Hattori T, Hayashi H, Mizutani Y, Toto M, Takii T, Yang D, Lee JC, Matsufuji S, Murakami Y, Chiba T, Onozaki K. Antiproliferative effect of IL-1 is mediated by p38 mitogen-activated protein kinase in human melanoma cell A375. J Immunol. 1999. 162:7434–7440.15. Tuo W, Harney JP, Bazer FW. Developmentally regulated expression of interleukin-1 beta by peri-implantation conceptuses in swine. J Reprod Immunol. 1996. 31:185–198.16. Cohen GM. Caspases: the executioners of apoptosis. J Biol Chem. 1997. 326:1–16.
Article17. Mehmet H. Caspases find a new place to hide. Nature. 2000. 403:29–30.
Article18. Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature. 1992. 30:768–774.19. Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett. 2001. 487:318–322.
Article20. Han Z, Pantazis P, Wyche JH, Kouttob N, Kidd VJ, Hendrickson EA. A Fas-associated death domain protein-dependent mechanism mediates the apoptotic action of non-steroidal anti-inflammatory drugs in the human leukemic Jurkat cell line. J Biol Chem. 2001. 276:38748–38754.
Article21. Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997. 3:614–620.
Article22. Weinmann P, Gaehtgens P, Walzog B. Bcl-xL-and Bax-α-mediated regulation of apoptosis of human neutrophils via caspase-3. Blood. 1999. 93:3106–3115.23. Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003. 22:4385–4399.
Article24. Sarin A, Haddad EK, Henkart PA. Caspase dependence of target cell damage induced by cytotoxic T lymphocytes. J Immunol. 1998. 161:2810–2816.25. van der Meer JW, Endres S, Lonnemann G, Cannon JG, Ikejima T, Okusawa S, Gelfand JA, Dinarello CA. Concentration of immunoreactive human tumor necrosis factor alpha produced by human mononuclear cells in vitro. J Leukocyte Biol. 1998. 43:216–223.26. Endres S, Ghorbani R, Lonnemann G, van der Meer JW, Dinarello CA. Measurement of immunoreactive interleukin-1β from human mononuclear cell: optimization of recovery, intransubject consistency, and comparison with interleukin-1α and tumor necrosis factor. Clin Immunol Immunopathol. 1988. 49:424–438.27. Decker P, Isenberg D, Muller S. Inhibition of caspase-3-mediated poly (ADP-ribose) polymerase (PARP) apoptotic cleavage by human PARP autoantibodies and effect on cells undergoing apoptosis. J Biol Chem. 2000. 275:9043–9046.28. Chen DX, Stetler RA, Cao GD. Characterization of the rat DNA fragmentation factor 35/inhibitor of caspase-activated DNase (short form). J Biol Chem. 2000. 275:38508–38517.
Article29. Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL-1β-converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci USA. 2001. 98:13249–13254.
Article30. Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases on caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997. 272:31138–31148.31. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Manogion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Abersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999. 397:441–446.
Article32. Takuma K, Phuagphong P, Lee E, Enomoto R, Mori K, Boda A, Matsuda G. The nitric oxide donor NOC12 protects cultured astrocytes against apoptosis via a cGMP-dependent mechanism. Jpn J Pharmacol. 2002. 89:64–71.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Norcantharidin Induces Human Melanoma A375-S2 Cell Apoptosis through Mitochondrial and Caspase Pathways
- Effects of B-16 Melanoma Cells and Mycoplasma pneumoniae on the Induction of IL-1 beta, IL-2, IL-6, IL-10, IL-12, and TNF - alpha from Mouse Astrocytes
- The Central and Peripheral Production of Pro-inflammatory Cytokine, IL-1b after Immune and Stress Stimulation in Rats
- Mechanism of LPS Induced IL-6 Expression in the Growth Arrested Human Mesanigial Cells
- Polymorphisms of interleukin-1beta promoter in simple febrile seizures