Tuberc Respir Dis.
2007 Mar;62(3):197-202. 10.4046/trd.2007.62.3.197.
Effects of Nicotine, Cotinine and Benzopyrene as Smoke Components on the Expression of Antioxidants in Human Bronchial Epithelial Cells
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Hanyang University, Seoul, Korea. shindh@hanyang.ac.kr
- 2Department of Biochemistry and Molecular Biology, College of Medicine, Hanyang University, Seoul, Korea.
- KMID: 1518464
- DOI: http://doi.org/10.4046/trd.2007.62.3.197
Abstract
-
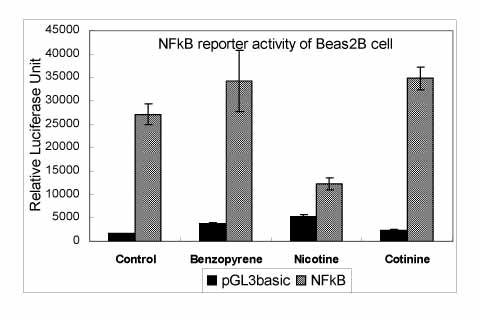
BACKGROUND: Cigarette smoking is an important risk factor for chronic bronchitis and COPD. Airway epithelial cells exposed to cigarette smoke components such as nicotine, cotinine and benzopyrene can generate reactive oxygen species (ROS) and be subject to oxidative stress. This oxidative stress can induce the inflammatory response in the lung by the oxidant itself or by the release of proinflammatory cytokines. It has been reported that nicotine stimulates ROS, which are associated with NF-kappaB.
METHODS
Beas2B cells were treated with nicotine, cotinine and benzopyrene. RT PCR was used to measure the expression of several antioxidant factors using the total RNA from the Beas2B cells. The level of superoxide dismutase(CuZnSOD), thioredoxin, glutathione reductase expression was examined.
RESULTS
0.5 to 4 hours after the benzopyrene, nicotine and cotinine theatments, the level of thioredoxin and glutathione reductase expression decreased. Longer exposure to these compounds for 24 to 72 hours inhibited the expression of most of these antioxidant factors.
CONCLUSION
During exposure to smoke compounds, thioredoxin and glutathione reductase are the key antioxidant factors induced sensitively between 0.5 and 4 hours but the levels these antioxidants decrease between 24 hour and 72hours.
Keyword
MeSH Terms
-
Antioxidants*
Bronchitis, Chronic
Cotinine*
Cytokines
Epithelial Cells*
Glutathione Reductase
Humans*
Lung
NF-kappa B
Nicotine*
Oxidative Stress
Polymerase Chain Reaction
Pulmonary Disease, Chronic Obstructive
Reactive Oxygen Species
Risk Factors
RNA
Smoke*
Smoking
Superoxides
Thioredoxins
Tobacco Products
Antioxidants
Cotinine
Cytokines
Glutathione Reductase
NF-kappa B
Nicotine
RNA
Reactive Oxygen Species
Smoke
Superoxides
Thioredoxins
Figure
Reference
-
1. Church T, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985. 64:111–126.2. Zang LY, Stone K, Pryor WA. Detection of free radicals in aqueous extracts of cigarette tar by electron spin resonance. Free Radic Biol Med. 1990. 8:275–279.3. Kinnula VL. Focus on antioxidant enzymes and antioxidant strategies in smoking related airway diseases. Thorax. 2005. 60:693–700.4. Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006. 533:222–239.5. Barr J, Sharma CS, Sarkar S, Wise K, Dong L, Periyakaruppan A, et al. Nicotine induces oxidative stress and activates nuclear transcription factor kappa B in rat mesencephalic cells. Mol Cell Biochem. 2006. [Epub ahead of print].6. Iho S, Tanaka Y, Takauji R, Kobayashi C, Muramatsu I, Iwasaki H, et al. Nicotine induces human neutrophils to produce IL-8 through the generation of peroxynitrite and subsequent activation of NFkappaB. J Leukoc Biol. 2003. 74:942–951.7. Di Stefano A, Caramori G, Oates T, Capelli A, Lusuardi M, Gnemmi I, et al. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J. 2000. 220:556–563.8. Briede JJ, Godschalk RW, Emans MT, De Kok TM, van Agen E, van Maanen J, et al. In vitro and in vivo studies on oxygen free radical and DNA adduct formation in rat lung and liver during benzo[a]pyrene metabolism. Free Radic Res. 2004. 38:995–1002.9. Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Lopez-Real AM, Labandeira-Garcia JL. Effects of (-)-nicotine and (-)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson's disease. Biochem Pharmacol. 2002. 64:125–135.10. Wei W, Kim Y, Boudreau N. Association of smoking with serum and dietary levels of antioxidants in adults: NHANES III, 1988-1994. Am J Public Health. 2001. 91:258–264.11. Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003. 167:1600–1619.12. Mates JM, Sanchez-Jimenez F. Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosci. 1999. 4:D339–D345.13. Nadeem A, Raj HG, Chhabra SK. Increased oxidative stress and altered levels of antioxidants in chronic obstructive pulmonary disease. Inflammation. 2005. 29:23–32.14. Sato A, Hara T, Nakamura H, Kato N, Hoshino Y, Kondo N, et al. Thioredoxin-1 suppresses systemic inflammatory responses against cigarette smoking. Antioxid Redox Signal. 2006. 8:1891–1896.15. Lakari E. Expression of oxidant and antioxidant enzymes in human lung and interstitial lung diseases. 2002. 1–86. Academic Dissertation to be presented with the assent of the Faculty of Medicine, University of Oulu, for public discussion in the Auditorium 1 of the University Hospital of Oulu, on April 19th.16. Solak ZA, Kabaroglu C, Cok G, Parildar Z, Bayindir U, Ozmen D, et al. Effect of different levels of cigarette smoking on lipid peroxidation, glutathione enzymes and paraoxonase 1 activity in healthy people. Clin Exp Med. 2005. 5:99–105.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between Passive Smoke and Urinary Cotinine Level
- Mitogenic effects of nicotine to human periodontal ligament(PDL) cells in vitro
- Environmental tobacco smoke and children's health
- An Analysis of Nicotine and its Metabolites in Plasma and Urine Samples of Tobacco Smokers and Nonsmokers by High Performance Liquid Chromatographic Method
- Effects of Antioxidant on Oxidative Stress and Autophagy in Bronchial Epithelial Cells Exposed to Particulate Matter and Cigarette Smoke Extract