Tuberc Respir Dis.
2007 Sep;63(3):242-250. 10.4046/trd.2007.63.3.242.
Microenvironments and Cellular Proliferation Affected by Oxygen Concentration in Non-Small Cell Lung Cancer Cell Line
- Affiliations
-
- 1Division of Allergy, Respiratory and Critical Care Medicine, Department of Internal Medicine, Chung Ang University College of Medicine, Seoul, Korea. iwpark@cau.ac.kr, basthma@hanmail.net
- KMID: 1518415
- DOI: http://doi.org/10.4046/trd.2007.63.3.242
Abstract
-
BACKGROUND: Abnormal angiogenesis can induce hypoxia within a highly proliferating tumor mass, and these hypoxic conditions can in turn create clinical problems, such as resistance to chemotherapy. However, the mechanism by which hypoxia induces these changes has not yet been determined. Therefore, this study was conducted to determine how hypoxia induces changes in cell viability and extracellular microenvironments in an in vitro culture system using non-small cell lung cancer cells.
METHODS
The non-small cell lung cancer cell line, A549 was cultured in DMEM or RPMI-1640 media that contained fetal bovine serum. A decrease in the oxygen tension of the media that contained the culture was then induced in a hypoxia microchamber using a CO2-N2 gas mixture. A gas analysis and an MTT assay were then conducted.
RESULTS
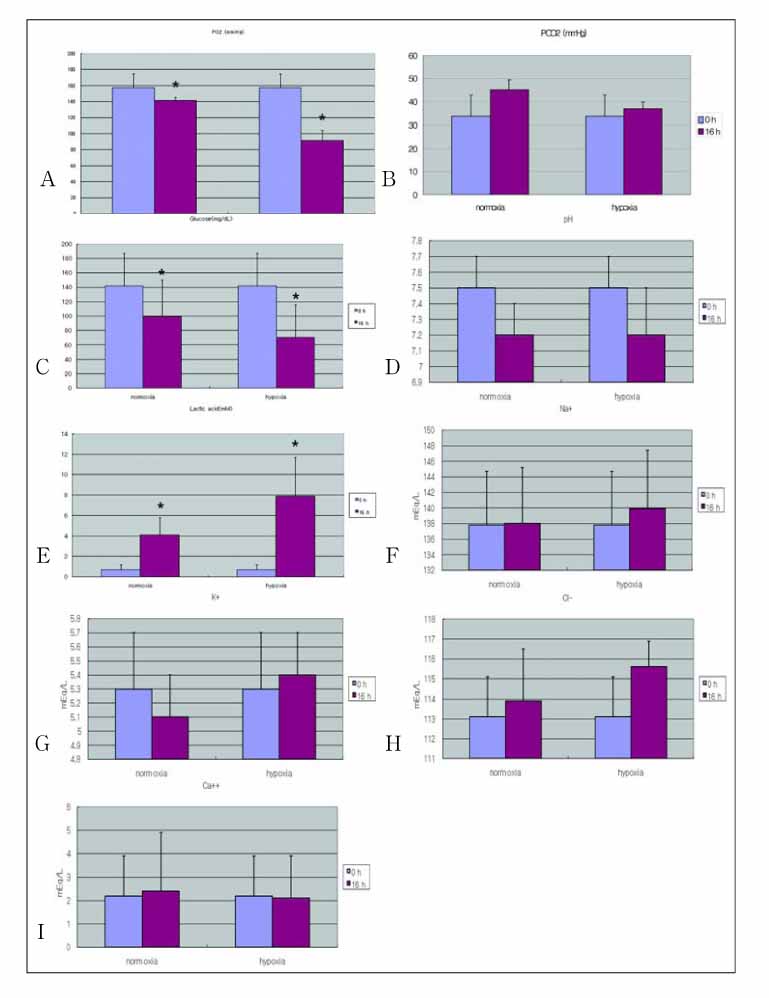
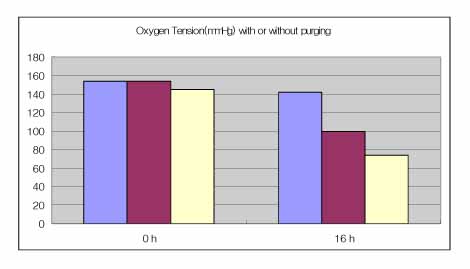
(1) The decrease in oxygen tension was checked the anaerobic gas mixture for 30 min and then reoxygenation was induced by adding a 5% CO2-room air gas mixture to the chamber. (2) Purging with the anaerobic gas mixture was found to decrease the further oxygen tension of cell culture media. (3) The low oxygen tension resulted in a low pH, lactic acidosis and a decreased glucose concentration in the media. (4) The decrease in glucose concentration that was observed as a result of hypoxia was markedly different when different types of media were evaluated. (5) The decrease in oxygen tension inhibited proliferation of A549 cells.
CONCLUSION
These data suggests that tumor hypoxia is associated with acidosis and hypoglycemia, which have been implicated in the development of resistance to chemotherapy and radiotherapy.
Keyword
MeSH Terms
Figure
Reference
-
1. O'Byrne KJ, Dalgleish AG, Browning MJ, Steward WP, Harris AL. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur J Cancer. 2000. 36:151–169.2. Brown JM. The hypoxic cell: a target for selective cancer therapy--eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1999. 59:5863–5870.3. Rofstad EK, Danielsen T. Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer. 1999. 80:1697–1707.4. Rofstad EK, Mathiesen B, Henriksen K, Kindem K, Galappathi K. The tumor bed effect: increased metastatic dissemination from hypoxia-induced up-regulation of metastasis-promoting gene products. Cancer Res. 2005. 65:2387–2396.5. Buchler P, Reber HA, Lavey RS, Tomlinson J, Buchler MW, Friess H, et al. Tumor hypoxia correlates with metastatic tumor growth of pancreatic cancer in an orthotopic murine model. J Surg Res. 2004. 120:295–303.6. Cangul H. Hypoxia upregulates the expression of the NDRG1 gene leading to its overexpression in various human cancers. BMC Genet. 2004. 5:27.7. Song X, Liu X, Chi W, Liu Y, Wei L, Wang X, et al. Hypoxia-induced resistance to cisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencing of HIF-1alpha gene. Cancer Chemother Pharmacol. 2006. 58:776–784.8. Koukourakis MI, Giatromanolaki A, Sivridis E, Fezoulidis I. Cancer vascularization: implications in radiotherapy? Int J Radiat Oncol Biol Phys. 2000. 48:545–553.9. Swinson DE, O'Byrne KJ. Interactions between hypoxia and epidermal growth factor receptor in non -small-cell lung cancer. Clin Lung Cancer. 2006. 7:250–256.10. Swinson DE, Jones JL, Cox G, Richardson D, Harris AL, O'Byrne KJ. Hypoxia-inducible factor-1 alpha in non small cell lung cancer: relation to growth factor, protease and apoptosis pathways. Int J Cancer. 2004. 111:43–50.11. Wang T, Niki T, Goto A, Ota S, Morikawa T, Nakamura Y, et al. Hypoxia increases the motility of lung adenocarcinoma cell line A549 via activation of the epidermal growth factor receptor pathway. Cancer Sci. 2007. 98:506–511.12. Koshikawa N, Takenaga K, Tagawa M, Sakiyama S. Therapeutic efficacy of the suicide gene driven by the promoter of vascular endothelial growth factor gene against hypoxic tumor cells. Cancer Res. 2000. 60:2936–2941.13. Li L, Yu J, Xing L, Ma K, Zhu H, Guo H, et al. Serial hypoxia imaging with 99mTc-HL91 SPECT to predict radiotherapy response in nonsmall cell lung cancer. Am J Clin Oncol. 2006. 29:628–633.14. Kobayashi S, Conforti L, Pun RY, Millhorn DE. Adenosine modulates hypoxia-induced responses in rat PC12 cells via the A2A receptor. J Physiol. 1998. 508(Pt 1):95–107.15. Ziemer LS, Lee WM, Vinogradov SA, Sehgal C, Wilson DF. Oxygen distribution in murine tumors: characterization using oxygen-dependent quenching of phosphorescence. J Appl Physiol. 2005. 98:1503–1510.16. Luo F, Liu X, Yan N, Li S, Cao G, Cheng Q, et al. Hypoxia-inducible transcription factor-1alpha promotes hypoxia-induced A549 apoptosis via a mechanism that involves the glycolysis pathway. BMC Cancer. 2006. 6:26.17. Hussain SP, Harris CC. Molecular epidemiology of human cancer. Recent Results Cancer Res. 1998. 154:22–36.18. Volm M, Koomagi R. Hypoxia-inducible factor (HIF-1) and its relationship to apoptosis and proliferation in lung cancer. Anticancer Res. 2000. 20:1527–1533.19. Lee SM, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Hypoxia confers protection against apoptosis via PI3K/Akt and ERK pathways in lung cancer cells. Cancer Lett. 2006. 242:231–238.20. Mairbaurl H, Wodopia R, Eckes S, Schulz S, Bartsch P. Impairment of cation transport in A549 cells and rat alveolar epithelial cells by hypoxia. Am J Physiol. 1997. 273:L797–L806.21. O'Kelly I, Peers C, Kemp PJ. O2-sensitive K+ channels in neuroepithelial body-derived small cell carcinoma cells of the human lung. Am J Physiol. 1998. 275:L709–L716.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Glutathione on High Dose Cisplatin-Induced Cellular Toxicity in Non-small Cell Lung Cancer Cell Lines
- Cell Death Induction Mechanism of Non-small Cell Lung Cancer Cell Line, NCI-H1703 by Docetaxel
- The effects of nutrient depleted microenvironments and delta-like 1 homologue (DLK1) on apoptosis in neuroblastoma
- Immunohistochemical Staining of Insulin-like Growth Factor-1 in Human Lung Cancer cells of NSCLC and SCLC
- Efficacy of Combination Chemotherapy with Paclitaxel and Cisplatin in Patients with Advanced Non-Small Cell Lung Cancer