Nutr Res Pract.
2010 Dec;4(6):455-461.
The effects of nutrient depleted microenvironments and delta-like 1 homologue (DLK1) on apoptosis in neuroblastoma
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, 11-1 Daehyun-dong, Seodaemun-gu, Seoul 120-750, Korea. yuri.kim@ewha.ac.kr
Abstract
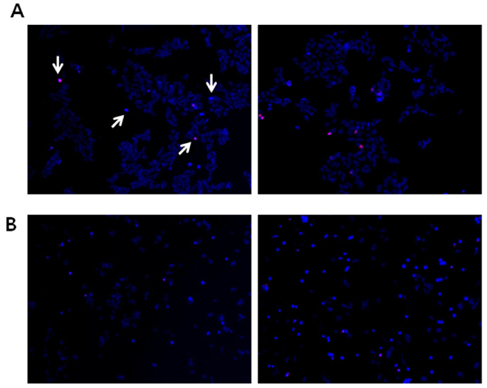
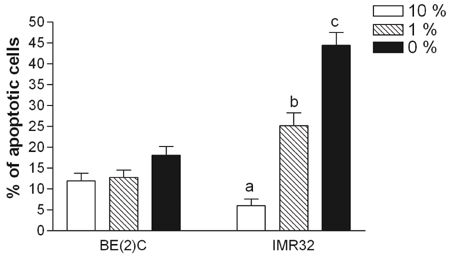
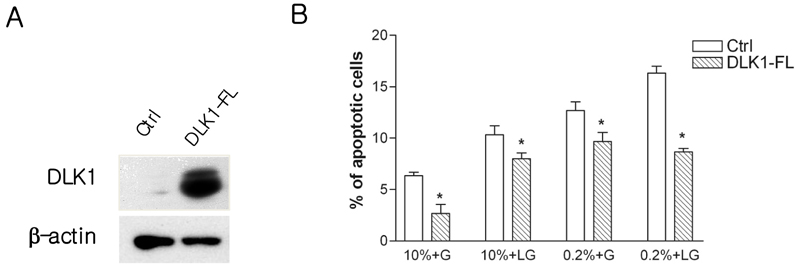
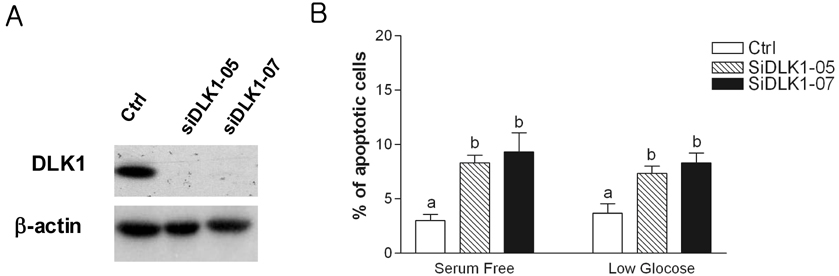
- The tumor microenvironment, particularly sufficient nutrition and oxygen supply, is important for tumor cell survival. Nutrition deprivation causes cancer cell death. Since apoptosis is a major mechanism of neuronal loss, we explored neuronal apoptosis in various microenvironment conditions employing neuroblastoma (NB) cells. To investigate the effects of tumor malignancy and differentiation on apoptosis, the cells were exposed to poor microenvironments characterized as serum-free, low-glucose, and hypoxia. Incubation of the cells in serum-free and low-glucose environments significantly increased apoptosis in less malignant and more differentiated N-type IMR32 cells, whereas more malignant and less differentiated I-type BE(2)C cells were not affected by those treatments. In contrast, hypoxia (1% O2) did not affect apoptosis despite cell malignancy. It is suggested that DLK1 constitutes an important stem cell pathway for regulating self-renewal, clonogenicity, and tumorigenicity. This raises questions about the role of DLK1 in the cellular resistance of cancer cells under poor microenvironments, which cancer cells normally encounter. In the present study, DLK1 overexpression resulted in marked protection from apoptosis induced by nutrient deprivation. This in vitro model demonstrated that increasing severity of nutrition deprivation and knock-down of DLK1 caused greater apoptotic death, which could be a useful strategy for targeted therapies in fighting NB as well as for evaluating how nutrient deprived cells respond to therapeutic manipulation.
Keyword
MeSH Terms
Figure
Reference
-
1. Shim H, Chun YS, Lewis BC, Dang CV. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci U S A. 1998. 95:1511–1516.2. Driscoll B, Buckley S, Barsky L, Weinberg K, Anderson KD, Warburton D. Abrogation of cyclin D1 expression predisposes lung cancer cells to serum deprivation-induced apoptosis. Am J Physiol. 1999. 276:L679–L687.3. Russo VC, Kobayashi K, Najdovska S, Baker NL, Werther GA. Neuronal protection from glucose deprivation via modulation of glucose transport and inhibition of apoptosis: a role for the insulin-like growth factor system. Brain Res. 2004. 1009:40–53.
Article4. Steller H. Mechanisms and genes of cellular suicide. Science. 1995. 267:1445–1449.
Article5. Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schutz G. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 2002. 31:47–54.
Article6. Ciani E, Guidi S, Della Valle G, Perini G, Bartesaghi R, Contestabile A. Nitric oxide protects neuroblastoma cells from apoptosis induced by serum deprivation through cAMP-response element-binding protein (CREB) activation. J Biol Chem. 2002. 277:49896–49902.
Article7. Ross RA, Biedler JL, Spengler BA. A role for distinct cell types in determining malignancy in human neuroblastoma cell lines and tumors. Cancer Lett. 2003. 197:35–39.
Article8. Walton JD, Kattan DR, Thomas SK, Spengler BA, Guo HF, Biedler JL, Cheung NK, Ross RA. Characteristics of stem cells from human neuroblastoma cell lines and in tumors. Neoplasia. 2004. 6:838–845.
Article9. Ciccarone V, Spengler BA, Meyers MB, Biedler JL, Ross RA. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res. 1989. 49:219–225.10. Rettig WJ, Spengler BA, Chesa PG, Old LJ, Biedler JL. Coordinate changes in neuronal phenotype and surface antigen expression in human neuroblastoma cell variants. Cancer Res. 1987. 47:1383–1389.11. Ross RA, Spengler BA, Domènech C, Porubcin M, Rettig WJ, Biedler JL. Human neuroblastoma I-type cells are malignant neural crest stem cells. Cell Growth Differ. 1995. 6:449–456.12. Laborda J. The role of the epidermal growth factor-like protein dlk in cell differentiation. Histol Histopathol. 2000. 15:119–129.13. Van Limpt VA, Chan AJ, Van Sluis PG, Caron HN, Van Noesel CJ, Versteeg R. High delta-like 1 expression in a subset of neuroblastoma cell lines corresponds to a differentiated chromaffin cell type. Int J Cancer. 2003. 105:61–69.
Article14. Laborda J, Sausville EA, Hoffman T, Notario V. dlk, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J Biol Chem. 1993. 268:3817–3820.
Article15. Yin D, Xie D, Sakajiri S, Miller CW, Zhu H, Popoviciu ML, Said JW, Black KL, Koeffler HP. DLK1: increased expression in gliomas and associated with oncogenic activities. Oncogene. 2006. 25:1852–1861.
Article16. Li L, Forman SJ, Bhatia R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene. 2005. 24:4472–4476.
Article17. Sakajiri S, OKelly J, Yin D, Miller CW, Hofmann WK, Oshimi K, Shih LY, Kim KH, Sul HS, Jensen CH, Teisner B, Kawamata N, Koeffler HP. Dlk1 in normal and abnormal hematopoiesis. Leukemia. 2005. 19:1404–1410.
Article18. Kim Y, Lin Q, Zelterman D, Yun Z. Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Res. 2009. 69:9271–9280.
Article19. Moore KA, Pytowski B, Witte L, Hicklin D, Lemischka IR. Hematopoietic activity of a stromal cell transmembrane protein containing epidermal growth factor-like repeat motifs. Proc Natl Acad Sci U S A. 1997. 94:4011–4016.
Article20. Ohno N, Izawa A, Hattori M, Kageyama R, Sudo T. dlk inhibits stem cell factor-induced colony formation of murine hematopoietic progenitors: Hes-1-independent effect. Stem Cells. 2001. 19:71–79.
Article21. Li L, Forman SJ, Bhatia R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene. 2005. 24:4472–4476.
Article22. Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Mol Cell Biol. 2005. 25:3040–3055.
Article23. Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996. 379:88–91.
Article24. Kaneta M, Osawa M, Sudo K, Nakauchi H, Farr AG, Takahama Y. A role for pref-1 and HES-1 in thymocyte development. J Immunol. 2000. 164:256–264.
Article25. Bauer SR, Ruiz-Hidalgo MJ, Rudikoff EK, Goldstein J, Laborda J. Modulated expression of the epidermal growth factor-like homeotic protein dlk influences stromal-cell-pre-B-cell interactions, stromal cell adipogenesis, and pre-B-cell interleukin-7 requirements. Mol Cell Biol. 1998. 18:5247–5255.
Article26. Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000. 25:294–297.
Article27. Birt DF, Yaktine A, Duysen E. Glucocorticoid mediation of dietary energy restriction inhibition of mouse skin carcinogenesis. J Nutr. 1999. 129:571S–574S.
Article28. Mukherjee P, El-Abbadi MM, Kasperzyk JL, Ranes MK, Seyfried TN. Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br J Cancer. 2002. 86:1615–1621.
Article29. Wei Z, Chen N, Guo H, Wang X, Xu F, Ren Q, Lu S, Liu B, Zhang L, Zhao H. Bone marrow mesenchymal stem cells from leukemia patients inhibit growth and apoptosis in serum-deprived K562 cells. J Exp Clin Cancer Res. 2009. 28:141.
Article30. Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death. Br J Cancer. 2002. 87:805–812.
Article31. Jögi A, Vallon-Christersson J, Holmquist L, Axelson H, Borg A, Påhlman S. Human neuroblastoma cells exposed to hypoxia: induction of genes associated with growth, survival, and aggressive behavior. Exp Cell Res. 2004. 295:469–487.
Article32. Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999. 17:2264–2279.
Article33. Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003. 3:203–216.
Article34. de Tudela MV, Delgado-Esteban M, Cuende J, Bolaños JP, Almeida A. Human neuroblastoma cells with MYCN amplification are selectively resistant to oxidative stress by transcriptionally up-regulating glutamate cysteine ligase. J Neurochem. 2010. 113:819–825.
Article35. Qi X, Chen Z, Liu D, Cen J, Gu M. Expression of Dlk1 gene in myelodysplastic syndrome determined by microarray, and its effects on leukemia cells. Int J Mol Med. 2008. 22:61–68.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of retinoic acid and delta-like 1 homologue (DLK1) on differentiation in neuroblastoma
- Delta-like Factor 1 as a Possible Therapeutic Target for Sarcomas
- Protective Effect of Delta-Like 1 Homolog Against Muscular Atrophy in a Mouse Model
- Correlation between Expression of CD44, Bcl-2, PCNA, and Apoptosis in Neuroblastoma According to Shimada Histology
- Kisspeptin and DLK1 levels for monitoring treatment of girls with central precocious puberty