Nutr Res Pract.
2010 Aug;4(4):276-282.
Effect of retinoic acid and delta-like 1 homologue (DLK1) on differentiation in neuroblastoma
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, 11-1 Daehyun-dong, Seodaemun-gu, Seoul 120-750, Korea. yuri.kim@ewha.ac.kr
Abstract
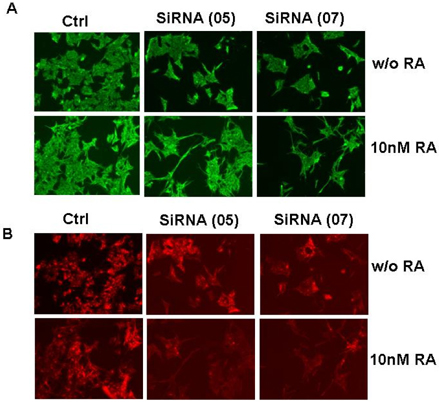
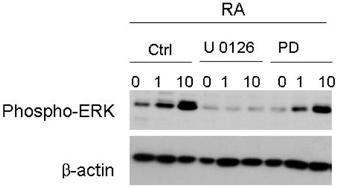
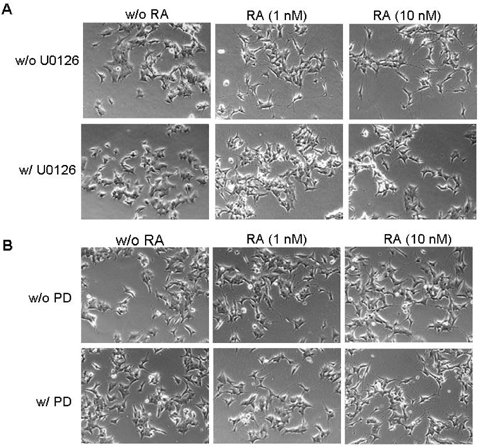
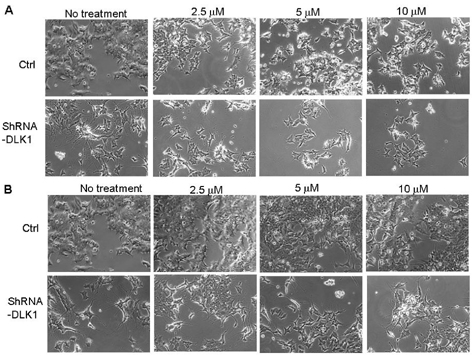
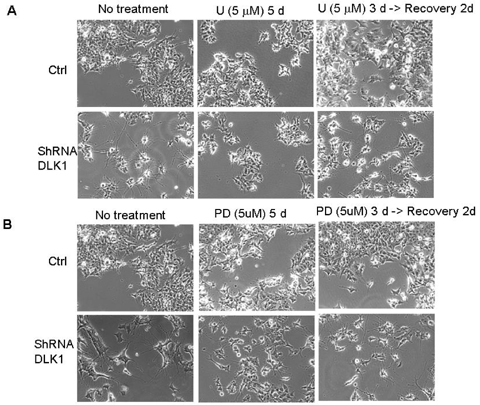
- The principal objective of this study was to evaluate the chemopreventive and therapeutic effects of a combination of all-trans-retinoic acid (RA) and knockdown of delta-like 1 homologue (Drosophila) (DLK1) on neuroblastoma, the most common malignant disease in children. As unfavorable neuroblastoma is poorly differentiated, neuroblastoma cell was induced differentiation by RA or DLK1 knockdown. Neuroblastoma cells showed elongated neurite growth, a hallmark of neuronal differentiation at various doses of RA, as well as by DLK1 knockdown. In order to determine whether or not a combination of RA and DLK1 knockdown exerts a greater chemotherapeutic effect on neuroblastoma, cells were incubated at 10 nM RA after being transfected with SiRNA-DLK1. Neuronal differentiation was increased more by a combination of RA and DLK1 knockdown than by single treatment. Additionally, in order to assess the signal pathway of neuroblastoma differentiation induced by RA and DLK1 knockdown, treatment with the specific MEK/ERK inhibitors, U0126 and PD 98059, was applied to differentiated neuroblastoma cells. Differentiation induced by RA and DLK1 knockdown increased ERK phosphorylation. The MEK/ERK inhibitor U0126 completely inhibited neuronal differentiation induced by both RA and DLK1 knockdown, whereas PD98059 partially blocked neuronal differentiation. After the withdrawal of inhibitors, cellular differentiation was fully recovered. This study is, to the best of our knowledge, the first to demonstrate that the specific inhibitors of the MEK/ERK pathway, U0126 and PD98059, exert differential effects on the ERK phosphorylation induced by RA or DLK1 knockdown. Based on the observations of this study, it can be concluded that a combination of RA and DLK1 knockdown increases neuronal differentiation for the control of the malignant growth of human neuroblastomas, and also that both MEK1 and MEK2 are required for the differentiation induced by RA and DLK1 knockdown.
Keyword
MeSH Terms
Figure
Reference
-
1. Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999. 17:2264–2279.
Article2. Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003. 3:203–216.
Article3. Kushner BH. Neuroblastoma: a disease requiring a multitude of imaging studies. J Nucl Med. 2004. 45:1172–1188.4. De Preter K, Vandesompele J, Heimann P, Beckman S, Schramm A, Eggert A, Stallings RL, Benoit Y, Renard M, De Paepe A, Laureys G, Påhlman S, Speleman F. Human fetal neuroblast and neuroblastoma transcriptome analysis confirms neuroblast origin and highlights neuroblastoma candidate genes. Genome Biol. 2006. 7:R84.5. Vasudevan SA, Nuchtern JG, Shohet JM. Gene profiling of high risk neuroblastoma. World J Surg. 2005. 29:317–324.
Article6. Matthay KK, Haas-Kogan D, Constine LS. Halperin EC, Constine LS, Tarbell NJ, Kun LE, editors. Neuroblastoma. Pediatric Radiation Oncology. 2005. Philadelphia: Lippincott Williams & Wilkins;179–222.7. Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D. European Neuroblastoma Study Group. Children's Cancer and Leukaemia Group (CCLG formerly United Kingdom Children's Cancer Study Group). High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol. 2008. 9:247–256.
Article8. Smith MA, Anderson B. Where to next with retinoids for cancer therapy? Clin Cancer Res. 2001. 7:2955–2957.9. Goodman DS, Huang HS. Biosynthesis of vitamin a with rat intestinal enzymes. Science. 1965. 149:879–880.
Article10. von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem. 2000. 275:11915–11920.11. Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006. 66:606–630.
Article12. Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, Fey M, Rayon C, Huguet F, Sotto JJ, Gardin C, Makhoul PC, Travade P, Solary E, Fegueux N, Bordessoule D, Miguel JS, Link H, Desablens B, Stamatoullas A, Deconinck E, Maloisel F, Castaigne S, Preudhomme C, Degos L. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999. 94:1192–1200.
Article13. Fenaux P, Chevret S, Guerci A, Fegueux N, Dombret H, Thomas X, Sanz M, Link H, Maloisel F, Gardin C, Bordessoule D, Stoppa AM, Sadoun A, Muus P, Wandt H, Mineur P, Whittaker JA, Fey M, Daniel MT, Castaigne S, Degos L. European APL group. Long-term follow-up confirms the benefit of all-trans retinoic acid in acute promyelocytic leukemia. Leukemia. 2000. 14:1371–1377.
Article14. Laborda J. The role of the epidermal growth factor-like protein dlk in cell differentiation. Histol Histopathol. 2000. 15:119–129.15. Floridon C, Jensen CH, Thorsen P, Nielsen O, Sunde L, Westergaard JG, Thomsen SG, Teisner B. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000. 66:49–59.
Article16. Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993. 73:725–734.
Article17. Tornehave D, Jensen CH, Teisner B, Larsson LI. FA1 immunoreactivity in endocrine tumours and during development of the human fetal pancreas; negative correlation with glucagon expression. Histochem Cell Biol. 1996. 106:535–542.
Article18. Villena JA, Kim KH, Sul HS. Pref-1 and ADSF/resistin: two secreted factors inhibiting adipose tissue development. Horm Metab Res. 2002. 34:664–670.
Article19. Li L, Forman SJ, Bhatia R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene. 2005. 24:4472–4476.
Article20. Sakajiri S, O'Kelly J, Yin D, Miller CW, Hofmann WK, Oshimi K, Shih LY, Kim KH, Sul HS, Jensen CH, Teisner B, Kawamata N, Koeffler HP. Dlk1 in normal and abnormal hematopoiesis. Leukemia. 2005. 19:1404–1410.
Article21. Bauer SR, Ruiz-Hidalgo MJ, Rudikoff EK, Goldstein J, Laborda J. Modulated expression of the epidermal growth factor-like homeotic protein dlk influences stromal-cell-pre-B-cell interactions, stromal cell adipogenesis, and pre-B-cell interleukin-7 requirements. Mol Cell Biol. 1998. 18:5247–5255.
Article22. Kaneta M, Osawa M, Sudo K, Nakauchi H, Farr AG, Takahama Y. A role for pref-1 and HES-1 in thymocyte development. J Immunol. 2000. 164:256–264.
Article23. Van Limpt VA, Chan AJ, Van Sluis PG, Caron HN, Van Noesel CJ, Versteeg R. High delta-like 1 expression in a subset of neuroblastoma cell lines corresponds to a differentiated chromaffin cell type. Int J Cancer. 2003. 105:61–69.
Article24. Laborda J, Sausville EA, Hoffman T, Notario V. dlk, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J Biol Chem. 1993. 268:3817–3820.
Article25. Yin D, Xie D, Sakajiri S, Miller CW, Zhu H, Popoviciu ML, Said JW, Black KL, Koeffler HP. DLK1: increased expression in gliomas and associated with oncogenic activities. Oncogene. 2006. 25:1852–1861.
Article26. Kim Y, Lin Q, Zelterman D, Yun Z. Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Res. 2009. 69:9271–9280.
Article27. Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995. 80:225–236.
Article28. Berwick DC, Calissano M, Corness JD, Cook SJ, Latchman DS. Regulation of Brn-3a N-terminal transcriptional activity by MEK1/2-ERK1/2 signalling in neural differentiation. Brain Res. 2009. 1256:8–18.
Article29. Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995. 270:27489–27494.
Article30. Cuenda A, Alessi DR. Use of kinase inhibitors to dissect signaling pathways. Methods Mol Biol. 2000. 99:161–175.
Article31. Ahn NG, Nahreini TS, Tolwinski NS, Resing KA. Pharmacologic inhibitors of MKK1 and MKK2. Methods Enzymol. 2001. 332:417–431.
Article32. Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Mol Cell Biol. 2005. 25:3040–3055.
Article33. Katayama K, Wada K, Miyoshi H, Ohashi K, Tachibana M, Furuki R, Mizuguchi H, Hayakawa T, Nakajima A, Kadowaki T, Tsutsumi Y, Nakagawa S, Kamisaki Y, Mayumi T. RNA interfering approach for clarifying the PPARgamma pathway using lentiviral vector expressing short hairpin RNA. FEBS Lett. 2004. 560:178–182.
Article34. De los Santos M, Zambrano A, Aranda A. Combined effects of retinoic acid and histone deacetylase inhibitors on human neuroblastoma SH-SY5Y cells. Mol Cancer Ther. 2007. 6:1425–1432.
Article35. Natale RB. Dual targeting of the vascular endothelial growth factor receptor and epidermal growth factor receptor pathways with vandetinib (ZD6474) in patients with advanced or metastatic non-small cell lung cancer. J Thorac Oncol. 2008. 3:S128–S130.
Article36. Miller VA, Benedetti FM, Rigas JR, Verret AL, Pfister DG, Straus D, Kris MG, Crisp M, Heyman R, Loewen GR, Truglia JA, Warrell RP Jr. Initial clinical trial of a selective retinoid X receptor ligand, LGD1069. J Clin Oncol. 1997. 15:790–795.
Article37. Shalinsky DR, Bischoff ED, Gregory ML, Lamph WW, Heyman RA, Hayes JS, Thomazy V, Davies PJ. Enhanced antitumor efficacy of cisplatin in combination with ALRT1057 (9-cis retinoic acid) in human oral squamous carcinoma xenografts in nude mice. Clin Cancer Res. 1996. 2:511–520.38. Laborda J. The role of the epidermal growth factor-like protein dlk in cell differentiation. Histol Histopathol. 2000. 15:119–129.39. Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000. 351:95–105.
Article40. Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998. 273:18623–18632.
Article41. Dang ZC, Lowik CW. Differential effects of PD98059 and U0126 on osteogenesis and adipogenesis. J Cell Biochem. 2004. 92:525–533.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of nutrient depleted microenvironments and delta-like 1 homologue (DLK1) on apoptosis in neuroblastoma
- Protective Effect of Delta-Like 1 Homolog Against Muscular Atrophy in a Mouse Model
- A Case of Acute Pancreatitis in a Neuroblastoma Patient after Retinoic Acid Therapy
- The Effect of Retinoids in Medulloblastoma Cell Culture
- Delta-like Factor 1 as a Possible Therapeutic Target for Sarcomas