J Bacteriol Virol.
2007 Dec;37(4):241-248. 10.4167/jbv.2007.37.4.241.
The Expression of TLR3 and Cytokines Induced by Poly I:C in Human Retinal Pigment Epithelial Cells
- Affiliations
-
- 1Department of Microbiology, Institute of Basic Medical Science, Yonsei University, Wonju College of Medicine, Il San Dong 162, Republic of Korea. joopark@yonsei.ac.kr
- 2Woori Eye Clinic, Joongang Dong, Wonju, Gangwon-do, 220-701, Republic of Korea.
- KMID: 1513585
- DOI: http://doi.org/10.4167/jbv.2007.37.4.241
Abstract
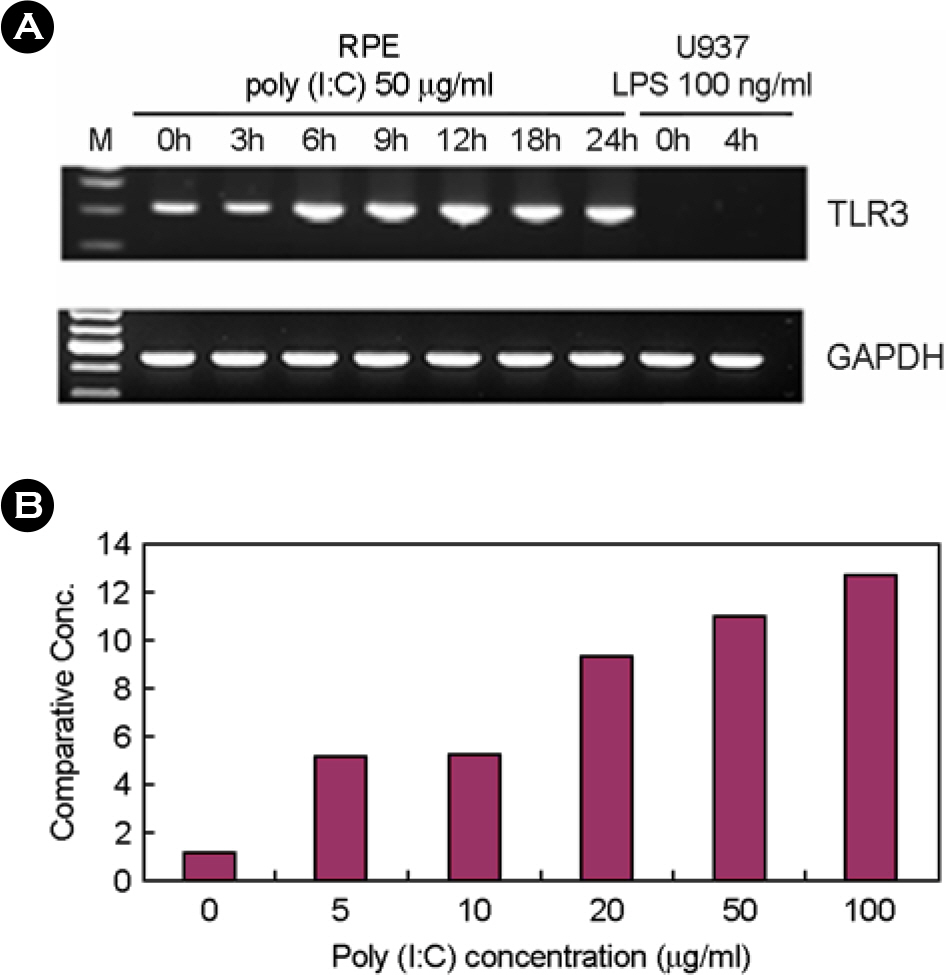
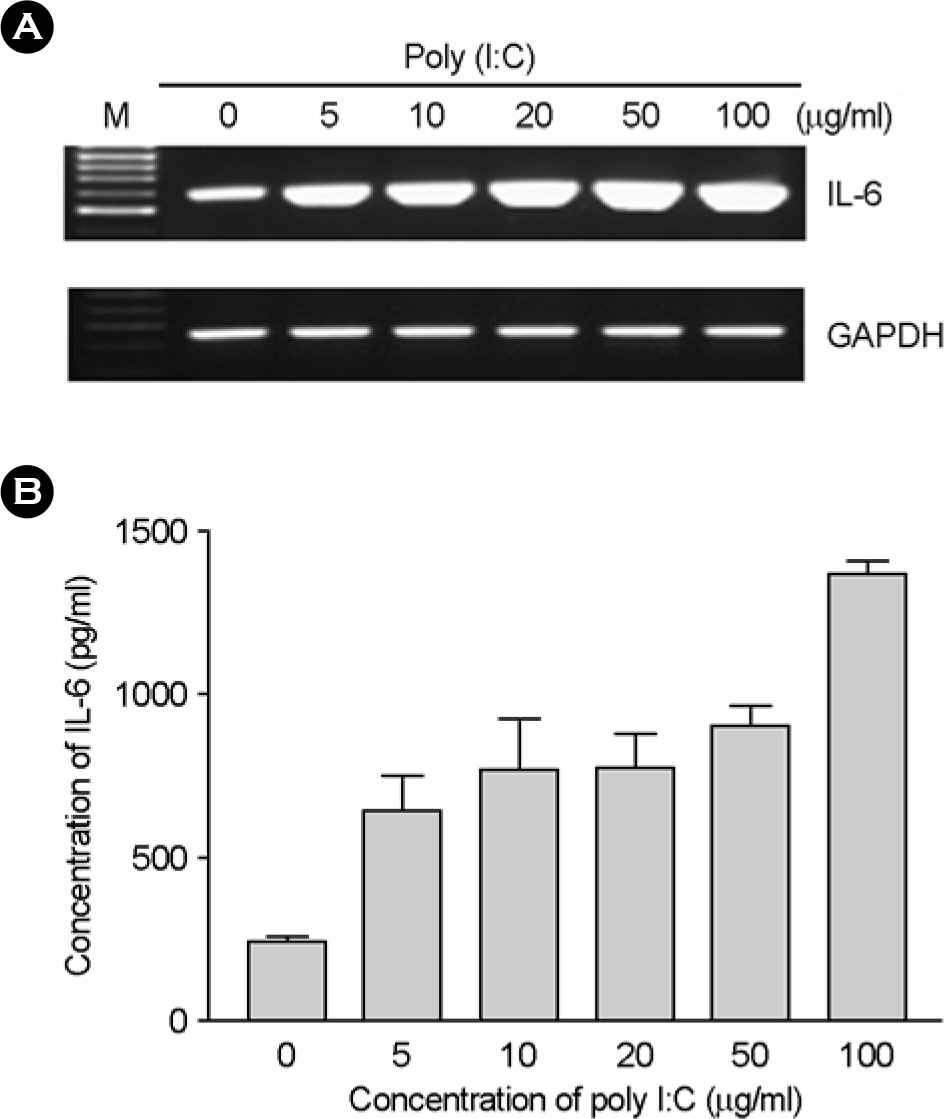
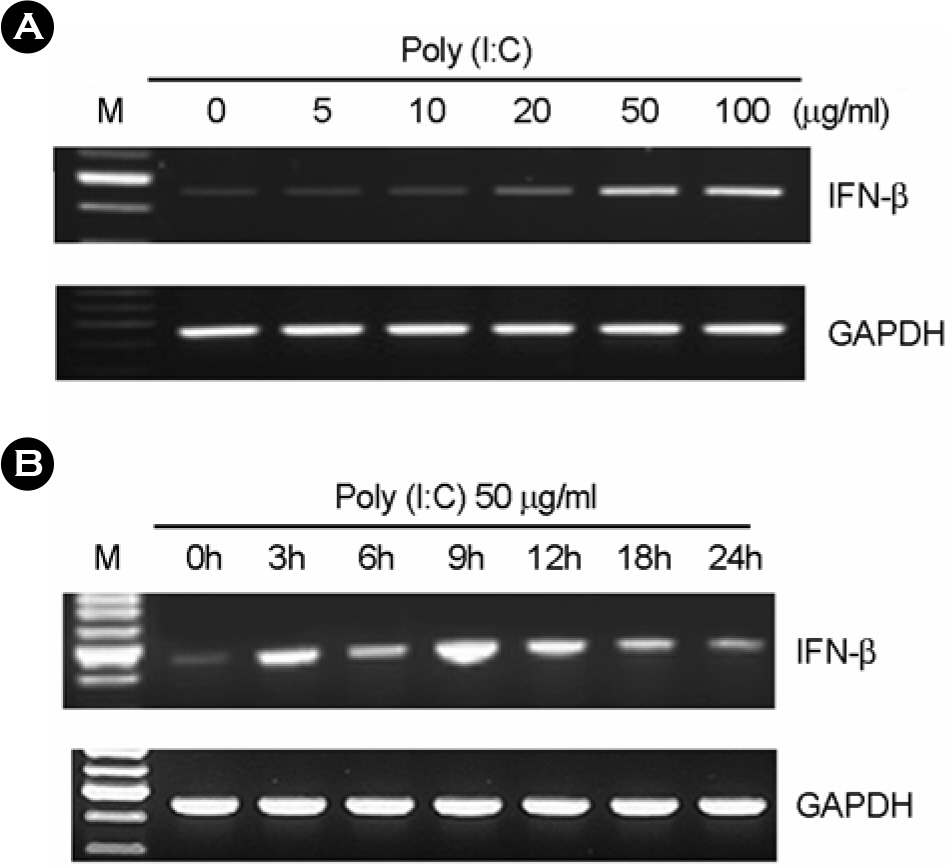
- In this study, we examined the expression of Toll-like receptor3 (TLR3) by human retinal pigment epithelial cells (RPE) and determined whether exposure to the TLR3 agonist polyinosinic-polycytidylic acid (poly I:C) would induced the expression of cytokines in these cells. RT-PCR revealed that TLR3 was constitutively expressed in human RPE, and its expression was increased by treatment with poly I:C. After treatment with poly I:C, we determined the expression levels of pro-inflammatory cytokines in human RPE using RT-PCR and ELISA. We demonstrated that poly I:C treatment increased the production of TNF-alpha, IL-6, and IL-8 in human RPE. Upon exposure to poly I:C, human RPE initiated antiviral response resulting in the induction of IFN-beta mRNA expression and 2',5'-oligoadenylate synthetase mRNA expression. These results suggest that human RPE may participate in ocular defense mechanism against viral infection through TLR3.
MeSH Terms
-
2',5'-Oligoadenylate Synthetase
Cytokines*
Enzyme-Linked Immunosorbent Assay
Epithelial Cells*
Humans*
Interferons
Interleukin-6
Interleukin-8
Poly I-C
Retinal Pigment Epithelium
Retinaldehyde*
RNA, Messenger
Tumor Necrosis Factor-alpha
2',5'-Oligoadenylate Synthetase
Cytokines
Interferons
Interleukin-6
Interleukin-8
Poly I-C
RNA, Messenger
Retinaldehyde
Tumor Necrosis Factor-alpha
Figure
Reference
-
References
1). Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 15:5–11. 2003.
Article2). Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 90:103–108. 2006.
Article3). Choi SJ, Lee KH, Park SJ, Kim J, Kim SK, Park JY. The Expression Pattern of Toll-like receptor (TLR) and Cytokine production to TLR Agonists in Human Retinal Pigment Epithlial Cells. J Bacteriol Virol. 37:119–128. 2007.4). Detrick B, Rhame J, Wang Y, Nagineni CN, Hooks JJ. Cytomegalovirus replication in human retinal pigment epithelial cells. Altered expression of viral early proteins. Invest Ophthalmol Vis Sci. 37:814–825. 1996.5). Elner SG, Petty HR, Elner VM, Yoshida A, Bian Z-M, Yang DL, Kindezelskii AL. TLR4 mediates human retinal pigment epithelial endotoxin binding and cytokine expression. Trans Am Ophthalmol Soc. 103:126–137. 2005.
Article6). Elner VM, Scales W, Elner SG, Danforth J, Kunkel SL, Strieter RM. Interleukin-6 (IL-6) gene expression and secretion by cytokine-stimulated human retinal pigment epithelial cells. Exp Eye Res. 54:361–368. 1992.
Article7). Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 3:196–200. 2002.
Article8). Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745. 2000.
Article9). Holtkamp GM, Kijlstra A, Peek R, de Vos AF. Retinal pigment epithelim-immune system interactions: Cytokine production and cytokine-induced changes. Prog Retin Eye Res. 20:29–48. 2001.10). Holtkamp GM, Van Rossem M, De Vos AF, Willekens B, Peek R, Kijlstra A. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol. 112:34–43. 1998.
Article11). Janeway CA Jr, Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin Immunol. 10:349–350. 1998.12). Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 3:499. 2002.
Article13). Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 117:11–21. 2006.
Article14). Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 153:7–15. 2004.
Article15). Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 14:432–436. 2002.
Article16). Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, Cottam HB. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci USA. 100:6646–6651. 2003.
Article17). Li XL, Blackford JA, Judge CS, Liu M, Xiao W, Kalvakolanu DV, Hassel BA. RNase-L-dependent destabilization of interferon-induced mRNAs. A role for the 2–5A system in attenuation of the interferon response. J Biol Chem. 275:8880–8888. 2000.18). Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, Carroll JD, Espevik T, Ingalls RR, Radolf JD, Golenbock DT. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 274:33419–33425. 1999.
Article19). Matsumoto M, Funami K, Oshimi H, Seya T. Toll-like receptor 3: A link between Toll-like receptor, interferon and viruses. Microbiol Immunol. 48:147–154. 2004.
Article20). Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 388:394–397. 1997.
Article21). Muzio M, Bosisio D, Polentarutti N, D'Amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton-Rol G, Ruco LP, Allavena P. and others:. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 164:5998–6004. 2000.
Article22). Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JFA. A family of human receptors structually related to Drosophila Toll. Proc Natl Acad Sci USA. 95:588–593. 1998.23). Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 14:778–809. 2001.
Article24). Sen GC, Sarkar SN. Transcriptional signaling by double-stranded RNA: role of TLR3. Cytokine Growth Factor Rev. 16:1–14. 2005.
Article25). Song PI, Abraham TA, Park YM, Zivony AS, Harten B, Edelhauser HF, Ward SL, Armstrong CA, Ansel JC. The expression of functional LPS receptor proteins CD14 and Toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 42:2867–2877. 2001.26). Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 16:3–9. 2004.
Article27). Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 21:335–376. 2003.
Article28). Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 42:392–403. 1969.
Article29). Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 168:554–561. 2002.
Article30). Zhou A, Hassel BA, Silverman RH. Expression cloning of 2–5A-dependent RNAase: a uniquely regulated mediator og interferon action. Cell. 72:753–765. 1993.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Protective Role of TLR3 and TLR9 Ligands in Human Pharyngeal Epithelial Cells Infected with Influenza A Virus
- Sensitivity of CD95-induced apoptosis in different proliferative status of human retinal pigment epithelial cells
- Growth Patterns of Human Retinal Pigment Epithelium in Vitro
- The Effect of 5-Fluorouraci1 on the Activity of the Retinal Pigment Epithelium in Vitro
- The Effects of Glucose Concentrations on Reactive Oxygen production and Cellular Activity in Retinal Pigment Epithelial Cells