J Bacteriol Virol.
2007 Dec;37(4):203-212. 10.4167/jbv.2007.37.4.203.
Proteomic Analysis of Helicobacter pylori Whole Cell Proteins using the Narrow Range IPG Strips
- Affiliations
-
- 1Department of Microbiology, Gyeongsang National University School of Medicine, Jinju, Gyeong-Nam 660-751, Republic of Korea. khrhee@gaechuk.gsnu.ac.kr
- 2Department of Pediatrics, Gyeongsang National University School of Medicine, Jinju, Gyeong-Nam 660-751, Republic of Korea.
- 3Institute of Health Science, Gyeongsang National University, Jinju, Gyeong-Nam 660-751, Republic of Korea.
- 4Research Institute of Life Science, Gyeongsang National University, Jinju, Gyeong-Nam 660-701, Republic of Korea.
- KMID: 1513579
- DOI: http://doi.org/10.4167/jbv.2007.37.4.203
Abstract
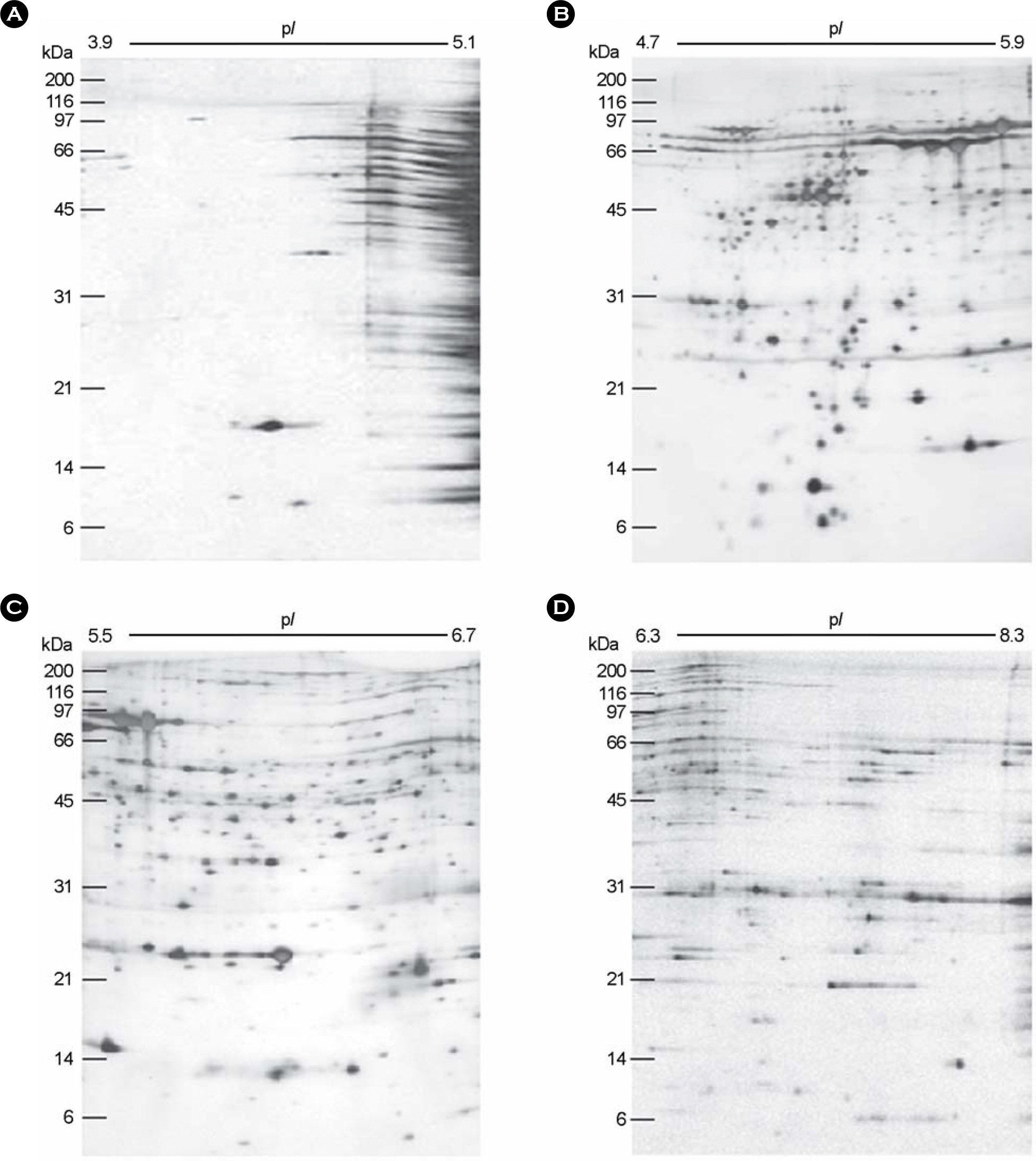
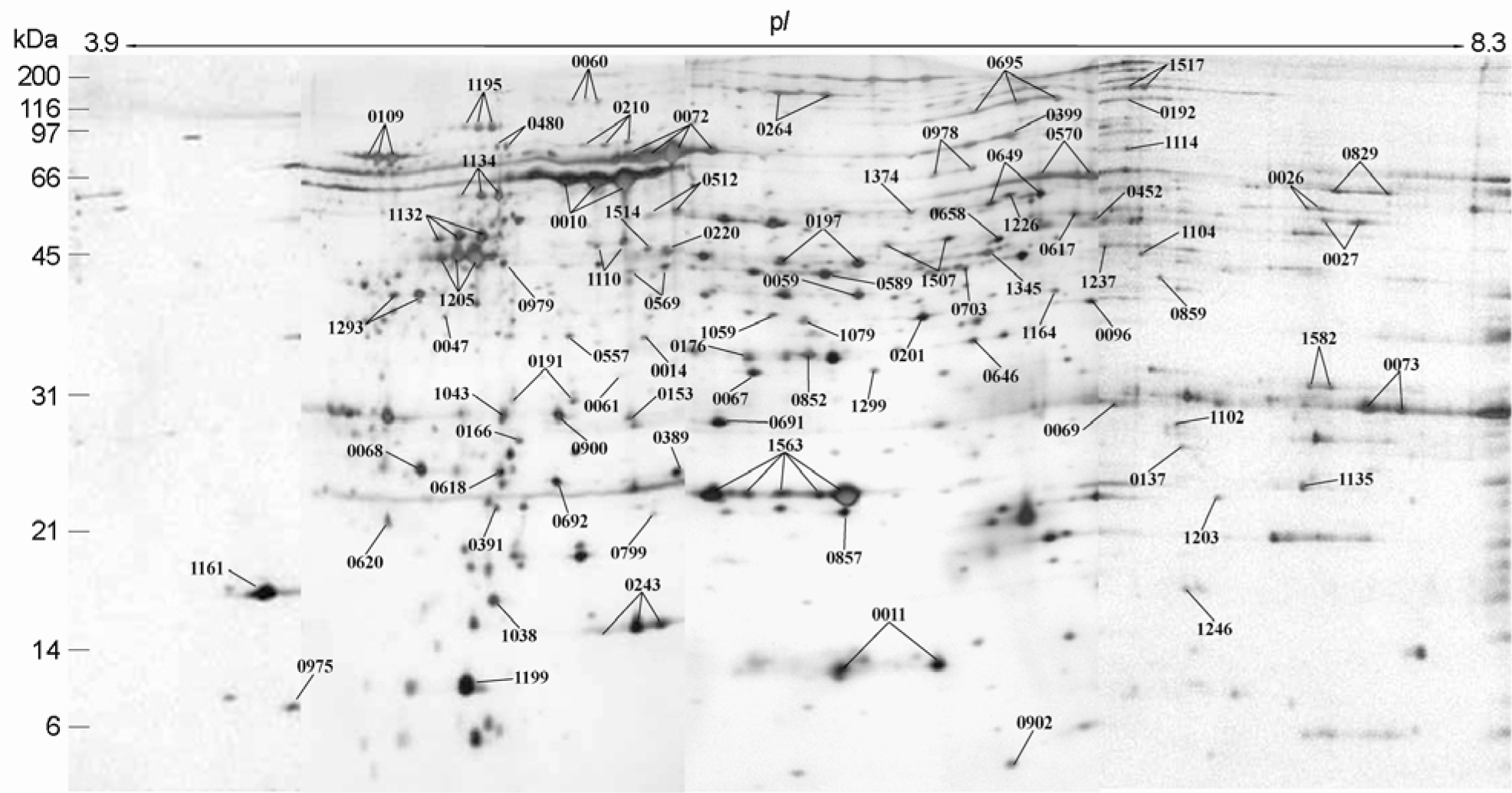
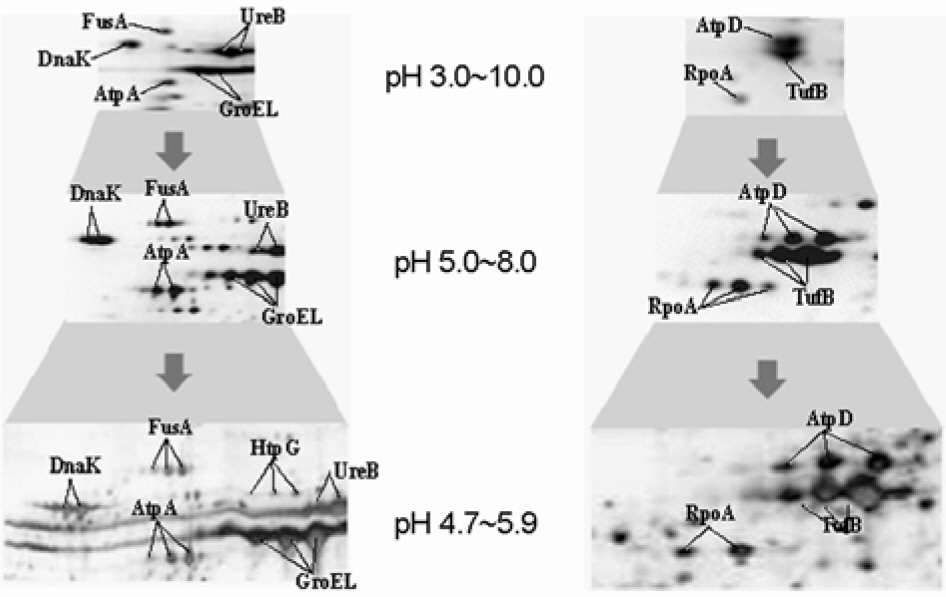
- It has been reported that most of Helicobacter pylori proteome components appear so crowded in the region of pH 4.5~8.0 that a lot of them were inseparable in 2-DE using the broad range IPG strip. Therefore, inseparable protein spots in 2-DE profiles have to be apart from each other for improving the protein identification. Here, we attempt to examine the usability of the narrow range IPG strips for separating close spots in the broad range IPG strip at proteomic analysis of H. pylori. The whole cell proteins of H. pylori strain 26695 were separated by narrow range IPG strips (pI 3.9~5.1, 4.7~5.9, 5.5~6.7, and 6.3~8.3, respectively), followed by SDS-PAGE, and visualized by silver staining, showing that the distances between spots were widened and the total number of detectable spots was increased. Resolved protein spots were identified by the peptide fingerprinting using MALDI-TOF-MS. As a result, 87 expressed proteins were identified by the peptide fingerprinting. Of them, 23 proteins, including hydrogenase expression/formation protein, purine-binding chemotaxis protein, and ribosomal protein S6, have not been reported in the previous proteome studies of H. pylori. Thus, these results demonstrate that the high complexity proteome components could be effectively separated using the narrow range IPG strips, which might be helpful to strengthen the contents of the master protein map of the H. pylori reference strain.
MeSH Terms
Figure
Cited by 2 articles
-
Helicobacter pylori: Bacterial Strategy for Incipient Stage and Persistent Colonization in Human Gastric Niches
Kwang-Ho Rhee, Jin-Sik Park, Myung-Je Cho
Yonsei Med J. 2014;55(6):1453-1466. doi: 10.3349/ymj.2014.55.6.1453.Proteomic Analysis of Thiol-active Proteins of Helicobacter pylori 26695
Jeong-Won Park, Jae-Young Song, Hyang-Ran Hwang, Hee-Jin Park, Hee-Shang Youn, Ji-Hyun Seo, Hyung-Lyun Kang, Kon-Ho Lee, Seung-Chul Baik, Woo-Kon Lee, Myung-Je Cho, Kwang-Ho Rhee
J Bacteriol Virol. 2012;42(3):211-223. doi: 10.4167/jbv.2012.42.3.211.
Reference
-
References
1). Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, Carmel G, Tummino PJ, Caruso A, Uria-Nickelsen M, Mills DM, Ives C, Gibson R, Merberg D, Mills SD, Jiang Q, Taylor DE, Vovis GF, Trust TJ. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 14:176–180. 1999.2). Backert S, Kwok T, Schmid M, Selbach M, Moese S, Peek RM Jr, Konig W, Meyer TF, Jungblut PR. Subproteomes of soluble and structure-bound Helicobacter pylori proteins analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 5:1331–1345. 2005.3). Baik SC, Kim JB, Cho MJ, Kim YC, Park CG, Rhou HH, Choi HJ, Rhee KH. Prevalence of Helicobacter pylori infection among normal Korean adults. J Korean Soc Microbiol. 6:455–462. 1990.4). Baik SC, Kim KM, Song SM, Kim DS, Jun JS, Lee SG, Song JY, Park JU, Kang HL, Lee WK, Cho MJ, Youn HS, Ko GH, Rhee KH. Proteomic analysis of the sarcosine-insoluble outer membrane fraction of Helicobacter pylori strain 26695. J Bacteriol. 186:949–955. 2004.5). Baik SC, Yoon HS, Chung MH, Lee WK, Cho MJ, Ko KH, Park CK, Kasai H, Rhee KH. Increased oxidative DNA damage Helicobacter pylori-infected human gastric mucosa. Cancer Res. 56:1279–1282. 1996.6). Banatvala NK, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori. J Infect Dis. 168:219–221. 1993.7). Blaser MJ. Gastric Campylobacter-like organisms gastritis and peptic ulcer disease. Gastroenterology. 97:371–383. 1987.8). Blaser MJ. Helicobacter pylori and gastric diseases. Br Med J. 316:1507–1510. 1998.9). Cash P. Proteomics in medical microbiology. Electrophoresis. 21:1187–1201. 2000.
Article10). Cho MJ, Jeon BS, Park JW, Jung TS, Song JY, Lee WK, Choi YJ, Choi SH, Park SG, Park JU, Choe MY, Jung SA, Byun EY, Baik SC, Youn HS, Ko GH, Lim D, Rhee KH. Identifying the major proteome components of Helicobacter pylori strain 26695. Electrophoresis. 23:1161–1173. 2002.11). Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 10:720–741. 1997.12). Fischer W, Haas R. The RecA protein of Helicobacter pylori requires a posttranslational modification for full activity. J Bacteriol. 186:777–784. 2004.13). Forman D, Newell DG, Fullerton F, Yarnel JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. Br Med J. 302:1302–1305. 1991.14). Gharahdaghi F, Weinberg CR, Maagher DA, Imai BS, Mische SM. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis. 20:601–605. 1999.
Article15). Görg A, Obermaier C, Boguth G, Harder A, Scheibe B, Wildgruber R, Weiss W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 21:1037–1053. 2000.
Article16). Görg A, Obermaier C, Boguth G, Weiss W. Recent developments in two-dimensional gel electrophoresis with immobilized pH gradients: wide pH gradients up to pH 12, longer separation distances and simplified procedures. Electrophoresis. 20:712–717. 1999.
Article17). Humphery-Smith I, Cordwell SJ, Blackstock WP. Proteome research: complementarity and limitations with respect to the RNA and DNA worlds. Electrophoresis. 18:1217–1242. 1997.
Article18). Jungblut PR, Bumann D, Haas G, Zimmy-Arndt U, Holland P, Lamer S, Siejak F, Aebischer A, Meyer TF. Comparative proteome analysis of Helicobacter pylori. Mol Microbiol. 36:710–725. 2000.19). Kim H, Wu CA, Kim DY, Han YH, Ha SC, Kim CS, Suh SW, Kim KK. Crystallization and preliminary X-ray crystallographic study of UDP-glucose pyrophosphorylase (UGPase) from Helicobacter pylori. Acta Crystallogr D Biol Crystallogr. 60:1447–1449. 2004.20). Lee HW, Choe YH, Kim DK, Jung SY, Lee NG. Proteomic analysis of a ferric uptake regulator mutant of Helicobacter pylori: regulation of Helicobacter pylori gene expression by ferric uptake regulator and iron. Proteomics. 4:2014–2027. 2004.21). Lock RA, Cordwell SJ, Coombs GW, Walsh BJ, Forbes GM. Proteome analysis of Helicobacter pylori: major proteins of type strain NCTC11637. Pathol. 33:365–374. 2001.22). Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 16:1311–115. 1984.
Article23). McAtee CP, Hoffman PS, Berg DE. Identification of differentially regulated proteins in metronidozole resistant Helicobacter pylori by proteome techniques. Proteomics. 1:516–521. 2001.24). Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 325:1132–1136. 1991.25). O'Connell KL, Stults JT. Identification of mouse liver proteins on two-dimensional electrophoresis gels by matrix-assisted laser desorption/ionization mass spectrometry of in situ enzymatic digests. Electrophoresis. 18:349–359. 1997.26). Oh JD, Kling-Bäckhed H, Giannakis M, Xu J, Fulton RS, Fulton LA, Cordum HS, Wang C, Elliott G, Edwards J, Mardis ER, Engstrand LG, Gordon JI. The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc Natl Acad Sci U S A. 103:9999–10004. 2006.27). Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and lymphoma. N Engl J Med. 330:1132–1136. 1994.28). Peterson W. Helicobacter pylori and peptic ulcer disease. N Engl J Med. 324:1043–1048. 1991.29). Proteome 2D-PAGE database. http://www.mpiib-berlin.mpg.de/2D-PAGE/.30). Quadroni M, James P. Proteomics and automation. Electrophoresis. 20:664–677. 1999.
Article31). Rhee KH, Yoon HS, Baik SC, Lee WK, Cho MJ, Choi HJ, Maeng KY, Ko KW. Prevalence of Helicobacter pylori infection among normal Koreans. J Korean Soc Microbiol. 6:475–490. 1990.32). Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, Nelson K, Quackenbush J, Zhou L, Kirkness EF, Peterson S, Loftus B, Richardson D, Dodson R, Khalak HG, Glodek A, McKenney K, Fitzegerald LM, Lee N, Adams MD, Hickey EK, Berg DE, Gocayne JD, Utterback TR, Peterson JD, Kelley JM, Cotton MD, Weidman JM, Fujii C, Bowman C, Watthey L, Wallin E, Hayes WS, Borodovsky M, Karp PD, Smith HO, Fraser CM, Venter JC. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 7:539–547. 1997.33). Uwins C, Deitrich C, Argo E, Stewart E, Davidson I, Cash P. Growth-induced changes in the proteome of Helicobacter pylori. Electrophoresis. 27:1136–1146. 2006.34). Wildgruber R, Harder A, Obermaier C, Boguth G, Weiss W, Fey SJ, Larsen PM, Görg A. Towards higher resolution: two-dimensional electrophoresis of Saccharomyces cerevisiae proteins using overlapping narrow immobilized pH gradients. Electrophoresis. 21:2610–2616. 2000.35). Williams KL. Genomes and proteomes: towards a multidimensional view of biology. Electrophoresis. 20:678–688. 1999.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two-dimensional gel electrophoresis of Helicobacter pylori for proteomic analysis
- Proteomic analysis of Helicobacter pylori J99 Outer Membrane Protein by Tandem Mass Spectrometry
- Treatment of Helicobacter pylori infection in functional dyspepsia
- Perspective of Helicobacter pylori Research: Molecular Pathogenesis of Helicobacter pylori Virulence Factors
- Secreted Proteins of Helicobacter pylori Identified using Pulse-Chase Analysis