Immune Netw.
2013 Oct;13(5):177-183. 10.4110/in.2013.13.5.177.
Nanoparticle-Based Vaccine Delivery for Cancer Immunotherapy
- Affiliations
-
- 1Department of Immunology, School of Medicine, Konkuk University, Chungju 380-701, Korea. jungid@kku.ac.kr, kangiron@kku.ac.kr, hanhd@kku.ac.kr
- KMID: 1493611
- DOI: http://doi.org/10.4110/in.2013.13.5.177
Abstract
- Development of nano-sized carriers including nanoparticles, nanoemulsions or liposomes holds great potential for advanced delivery systems for cancer immunotherapy, as such nanostructures can be used to more effectively manipulate or deliver immunologically active components to specific target sites. Successful development of nanotechnology based platform in the field of immunotherapy will allow the application of vaccines, adjuvants and immunomodulatory drugs that improve clinical outcomes for immunological diseases. Here, we review current nanoparticle-based platforms in the efficacious delivery of vaccines in cancer immunotherapy.
Keyword
MeSH Terms
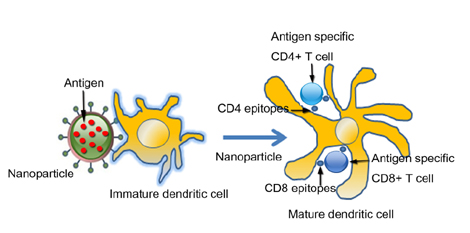
Figure
Cited by 2 articles
-
Far Beyond Cancer Immunotherapy: Reversion of Multi-Malignant Phenotypes of Immunotherapeutic-Resistant Cancer by Targeting the NANOG Signaling Axis
Se Jin Oh, Jaeyoon Lee, Yukang Kim, Kwon-Ho Song, Eunho Cho, Minsung Kim, Heejae Jung, Tae Woo Kim
Immune Netw. 2020;20(1):e7. doi: 10.4110/in.2020.20.e7.Far Beyond Cancer Immunotherapy: Reversion of Multi-Malignant Phenotypes of Immunotherapeutic-Resistant Cancer by Targeting the NANOG Signaling Axis
Se Jin Oh, Jaeyoon Lee, Yukang Kim, Kwon-Ho Song, Eunho Cho, Minsung Kim, Tae Woo Kim, Heejae Jung
Immune Netw. 2020;20(1):. doi: 10.4110/in.2020.20.e7.
Reference
-
1. Craparo EF, Bondi ML. Application of polymeric nanoparticles in immunotherapy. Curr Opin Allergy Clin Immunol. 2012; 12:658–664.
Article2. Smith DM, Simon JK, Baker JR Jr. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013; 13:592–605.
Article3. Serda RE. Particle platforms for cancer immunotherapy. Int J Nanomedicine. 2013; 8:1683–1696.
Article4. Rodriguez-Limas WA, Sekar K, Tyo KE. Virus-like particles: the future of microbial factories and cell-free systems as platforms for vaccine development. Curr Opin Biotechnol. 2013; In press: http://dx.doi.org/10.1016/j.copbio.5. Yuba E, Harada A, Sakanishi Y, Watarai S, Kono K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials. 2013; 34:3042–3052.
Article6. Syed S, Zubair A, Frieri M. Immune response to nanomaterials: implications for medicine and literature review. Curr Allergy Asthma Rep. 2013; 13:50–57.
Article7. Ditto AJ, Shah PN, Yun YH. Non-viral gene delivery using nanoparticles. Expert Opin Drug Deliv. 2009; 6:1149–1160.
Article8. Hua S, Cabot PJ. Targeted nanoparticles that mimic immune cells in pain control inducing analgesic and anti-inflammatory actions: a potential novel treatment of acute and chronic pain condition. Pain Physician. 2013; 16:E199–E216.9. Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol. 2013; 3:1–13. doi: 10.3389/fcimb.2013.00013.
Article10. Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur J Pharm Biopharm. 2013; In press: http://dx.doi.org/10.1016/j.ejpb.2013.07.002.
Article11. Pawar D, Mangal S, Goswami R, Jaganathan KS. Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: Effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur J Pharm Biopharm. 2013; In press: http://dx.doi.org/10.1016/j.ejpb.2013.06.017.
Article12. DeMuth PC, Moon JJ, Suh H, Hammond PT, Irvine DJ. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano. 2012; 6:8041–8051.
Article13. Lee JS, Kim DH, Lee CM, Ha TK, Noh KT, Park JW, Heo DR, Son KH, Jung ID, Lee EK, Shin YK, Ahn SC, Park YM. Deoxypodophyllotoxin induces a Th1 response and enhances the antitumor efficacy of a dendritic cell-based vaccine. Immune Netw. 2011; 11:79–94.
Article14. Noh KT, Shin SJ, Son KH, Jung ID, Kang HK, Lee SJ, Lee EK, Shin YK, You JC, Park YM. The Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein, a toll-like receptor 4 agonist, enhances dendritic cell-based cancer vaccine potency. Exp Mol Med. 2012; 44:340–349.
Article15. Cho NH, Cheong TC, Min JH, Wu JH, Lee SJ, Kim D, Yang JS, Kim S, Kim YK, Seong SY. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 2011; 6:675–682.
Article16. Noh YW, Hong JH, Shim SM, Park HS, Bae HH, Ryu EK, Hwang JH, Lee CH, Cho SH, Sung MH, Poo H, Lim YT. Polymer nanomicelles for efficient mucus delivery and antigen-specific high mucosal immunity. Angew Chem Int Ed Engl. 2013; 52:7684–7689.
Article17. Prasad S, Cody V, Saucier-Sawyer JK, Saltzman WM, Sasaki CT, Edelson RL, Birchall MA, Hanlon DJ. Polymer nanoparticles containing tumor lysates as antigen delivery vehicles for dendritic cell-based antitumor immunotherapy. Nanomedicine. 2011; 7:1–10.
Article18. Silva JM, Videira M, Gaspar R, Preat V, Florindo HF. Immune system targeting by biodegradable nanoparticles for cancer vaccines. J Control Release. 2013; 168:179–199.
Article19. Xiang SD, Wilson K, Day S, Fuchsberger M, Plebanski M. Methods of effective conjugation of antigens to nanoparticles as non-inflammatory vaccine carriers. Methods. 2013; 60:232–241.
Article20. Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies BJ, Marsland BJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc Natl Acad Sci USA. 2011; 108:E989–E997.
Article21. Andrade F, Rafael D, Videira M, Ferreira D, Sosnik A, Sarmento B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv Drug Deliv Rev. In press: http://dx.doi.org/10.1016/j.addr.2013.07.020.
Article22. Suh WH, Suslick KS, Stucky GD, Suh YH. Nanotechnology, nanotoxicology, and neuroscience. Prog Neurobiol. 2009; 87:133–170.
Article23. Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008; 83:761–769.
Article24. Kotagiri N, Lee JS, Kim JW. Selective pathogen targeting and macrophage evading carbon nanotubes through dextran sulfate coating and PEGylation for photothermal theranostics. J Biomed Nanotechnol. 2013; 9:1008–1016.
Article25. Kanekiyo M, Wei CJ, Yassine HM, McTamney PM, Boyington JC, Whittle JR, Rao SS, Kong WP, Wang L, Nabel GJ. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013; 499:102–106.
Article26. Perry JL, Reuter KG, Kai MP, Herlihy KP, Jones SW, Luft JC, Napier M, Bear JE, DeSimone JM. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012; 12:5304–5310.
Article27. Kim JH, Noh YW, Heo MB, Cho MY, Lim YT. Multifunctional hybrid nanoconjugates for efficient in vivo delivery of immunomodulating oligonucleotides and enhanced antitumor immunity. Angew Chem Int Ed Engl. 2012; 51:9670–9673.
Article28. Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Mark Saltzman W, Mellman I, Ledizet M, Fikrig E, Flavell RA, Fahmy TM. Inflammasomeactivating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009; 27:3013–3021.
Article29. Diwan M, Tafaghodi M, Samuel J. Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. J Control Release. 2002; 85:247–262.
Article30. Clawson C, Huang CT, Futalan D, Seible DM, Saenz R, Larsson M, Ma W, Minev B, Zhang F, Ozkan M, Ozkan C, Esener S, Messmer D. Delivery of a peptide via poly(D,L-lactic-co-glycolic) acid nanoparticles enhances its dendritic cell-stimulatory capacity. Nanomedicine. 2010; 6:651–661.
Article31. Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006; 117:78–88.
Article32. Liu SY, Wei W, Yue H, Ni DZ, Yue ZG, Wang S, Fu Q, Wang YQ, Ma GH, Su ZG. Nanoparticles-based multi-adjuvant whole cell tumor vaccine for cancer immunotherapy. Biomaterials. 2013; 34:8291–8300.
Article33. Shima F, Akagi T, Uto T, Akashi M. Manipulating the antigen-specific immune response by the hydrophobicity of amphiphilic poly(gamma-glutamic acid) nanoparticles. Biomaterials. 2013; 34:9709–9716.
Article34. Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007; 25:1159–1164.
Article35. Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010; 10:787–796.
Article36. Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005; 298:315–322.
Article37. Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm. 2007; 4:73–84.
Article38. Thurn KT, Brown E, Wu A, Vogt S, Lai B, Maser J, Paunesku T, Woloschak GE. Nanoparticles for applications in cellular imaging. Nanoscale Res Lett. 2007; 2:430–441.
Article39. Yan W, Chen W, Huang L. Reactive oxygen species play a central role in the activity of cationic liposome based cancer vaccine. J Control Release. 2008; 130:22–28.
Article40. Venkataraman S, Hedrick JL, Ong ZY, Yang C, Ee PL, Hammond PT, Yang YY. The effects of polymeric nanostructure shape on drug delivery. Adv Drug Deliv Rev. 2011; 63:1228–1246.
Article41. Florez L, Herrmann C, Cramer JM, Hauser CP, Koynov K, Landfester K, Crespy D, Mailander V. How shape influences uptake: interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small. 2012; 8:2222–2230.
Article42. Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008; 5:505–515.
Article43. Kelly C, Jefferies C, Cryan SA. Targeted liposomal drug delivery to monocytes and macrophages. J Drug Deliv. 2011; 2011:727241.
Article44. Nguyen DN, Mahon KP, Chikh G, Kim P, Chung H, Vicari AP, Love KT, Goldberg M, Chen S, Krieg AM, Chen J, Langer R, Anderson DG. Lipid-derived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc Natl Acad Sci USA. 2012; 109:E797–E803.
Article45. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003; 19:71–82.
Article46. Lee IH, Kwon HK, An S, Kim D, Kim S, Yu MK, Lee JH, Lee TS, Im SH, Jon S. Imageable antigen-presenting gold nanoparticle vaccines for effective cancer immunotherapy in vivo. Angew Chem Int Ed Engl. 2012; 51:8800–8805.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vaccine adjuvant materials for cancer immunotherapy and control of infectious disease
- Recombinant Bacille Calmette–Guérin for Immunotherapy in Nonmuscle Invasive Bladder Cancer
- Cancer Vaccines
- The Clinical Impact of the Dendritic Cell-based Cancer Vaccine: the Role in the Inflammatory Tumor Micro-environment
- Distinct features of dendritic cell-based immunotherapy as cancer vaccines