Immune Netw.
2010 Feb;10(1):5-14. 10.4110/in.2010.10.1.5.
Ginsan Enhances Humoral Antibody Response to Orally Delivered Antigen
- Affiliations
-
- 1Department of Microbiology, Dankook University College of Medicine, Cheonan 330-714, Korea.
- 2Department of Nursing, Chunnam Techno College, Gokseong 526-911, Korea.
- 3Laboratory of Immunology, Korea Institute of Radiological and Medical Sciences, Seoul 139-706, Korea.
- 4Mucosal Immunology Section, International Vaccine Institute, Seoul 151-919, Korea.
- 5Department of Microbiology, Chonnam National University Medical School, Gwangju 501-746, Korea. hclee@chonnam.ac.kr
- 6The Brain Korea 21 Project, Center for Biomedical Human Resources at Chonnam National University, Gwangju 501-746, Korea.
- KMID: 1456192
- DOI: http://doi.org/10.4110/in.2010.10.1.5
Abstract
- BACKGROUND
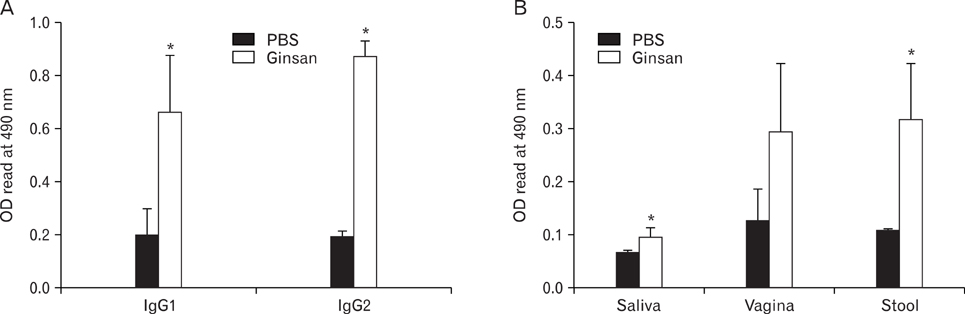
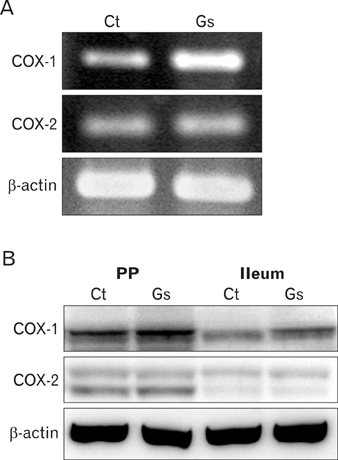
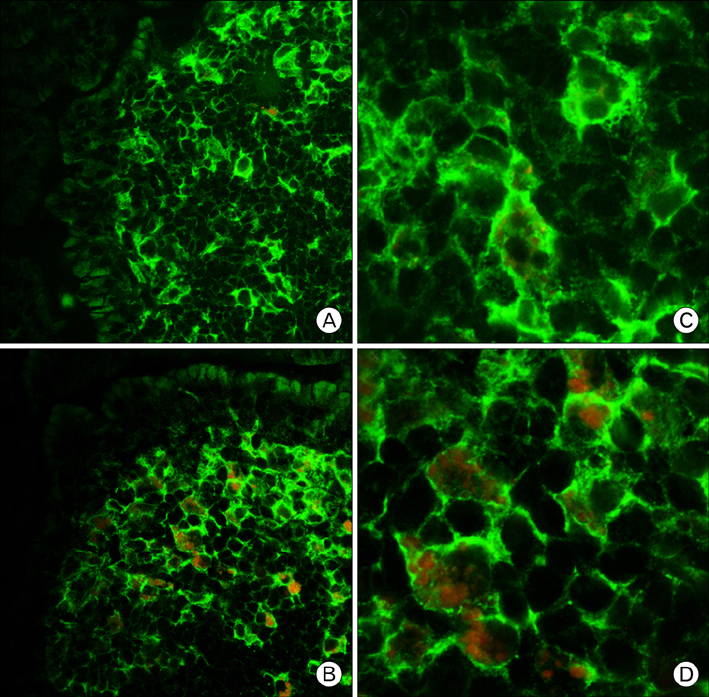
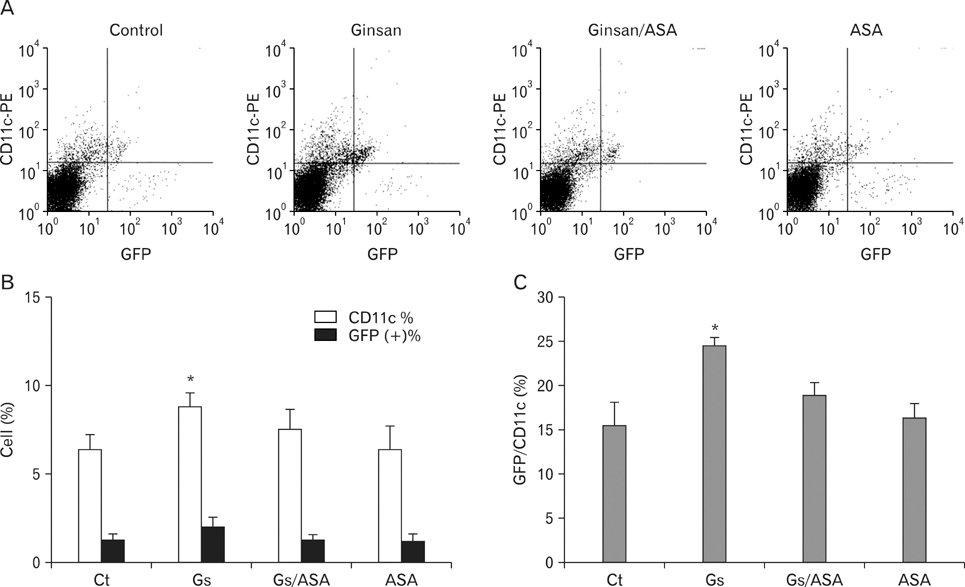
There have been several reports describing the capability of ginseng extracts as an adjuvant. In this study, we tested if ginsan, a polysaccharide extracted from Panax ginseng, was effective in enhancing antibody response to orally delivered Salmonella antigen. METHODS: Ginsan was treated before oral salmonella antigen administration. Salmonella specific antibody was determined by ELISA. mRNA expression was determined by RT-PCR. Cell migration was determined by confocal microscopy and flow cytometry. COX expression was detected by western blot. RESULTS: Ginsan treatment before oral Salmonella antigen delivery significantly increased both secretory and serum antibody production. Ginsan increased the expression of COX in the Peyer's patches. Various genes were screened and we found that CCL3 mRNA expression was increased in the Peyer's patch. Ginsan increased dendritic cells in the Peyer's patch and newly migrated dendritic cells were mostly found in the subepithelial dome region. When COX inhibitors were treated, the expression of CCL3 was reduced. COX inhibitor also antagonized both the migration of dendritic cells and the humoral immune response against oral Salmonella antigen. CONCLUSION: Ginsan effectively enhances the humoral immune response to orally delivered antigen, mediated by CCL3 via COX. Ginsan may serve as a potent vaccine suppliment for oral immunization.
Keyword
MeSH Terms
-
Antibody Formation
Blotting, Western
Cell Movement
Dendritic Cells
Enzyme-Linked Immunosorbent Assay
Flow Cytometry
Immunity, Humoral
Immunization
Microscopy, Confocal
Panax
Peyer's Patches
Polysaccharides
Prostaglandin-Endoperoxide Synthases
RNA, Messenger
Salmonella
Polysaccharides
Prostaglandin-Endoperoxide Synthases
RNA, Messenger
Figure
Reference
-
1. Plotkin SA. Vaccines: past, present and future. Nat Med. 2005. 11:4 Suppl. S5–S11.
Article2. Brokstad KA, Eriksson J, Cox RJ, Tynning T, Olofsson J, Jonsson R, Davidsson A. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J Infect Dis. 2002. 185:878–884.
Article3. Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev. 2000. 13:523–533.
Article4. Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002. 60:258–274.
Article5. Lu J, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009. 7:293–302.
Article6. Rivera E, Hu S, Concha C. Ginseng and aluminium hydroxide act synergistically as vaccine adjuvants. Vaccine. 2003. 21:1149–1157.
Article7. Hu S, Concha C, Lin F, Persson Waller K. Adjuvant effect of ginseng extracts on the immune responses to immunisation against Staphylococcus aureus in dairy cattle. Vet Immunol Immunopathol. 2003. 91:29–37.
Article8. Rivera E, Daggfeldt A, Hu S. Ginseng extract in aluminium hydroxide adjuvanted vaccines improves the antibody response of pigs to porcine parvovirus and Erysipelothrix rhusiopathiae. Vet Immunol Immunopathol. 2003. 91:19–27.
Article9. Scaglione F, Cattaneo G, Alessandria M, Cogo R. Efficacy and safety of the standardised Ginseng extract G115 for potentiating vaccination against the influenza syndrome and protection against the common cold [corrected]. Drugs Exp Clin Res. 1996. 22:65–72.10. Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS, Jeong G, Song JY. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006. 36:37–45.
Article11. Han Y, Son SJ, Akhalaia M, Platonov A, Son HJ, Lee KH, Yun YS, Song JY. Modulation of radiation-induced disturbances of antioxidant defense systems by ginsan. Evid Based Complement Alternat Med. 2005. 2:529–536.
Article12. Ivanova T, Han Y, Son HJ, Yun YS, Song JY. Antimutagenic effect of polysaccharide ginsan extracted from Panax ginseng. Food Chem Toxicol. 2006. 44:517–521.
Article13. Kim KH, Lee YS, Jung IS, Park SY, Chung HY, Lee IR, Yun YS. Acidic polysaccharide from Panax ginseng, ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Planta Med. 1998. 64:110–115.
Article14. Shin JY, Song JY, Yun YS, Yang HO, Rhee DK, Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002. 24:469–482.
Article15. Song JY, Han SK, Son EH, Pyo SN, Yun YS, Yi SY. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int Immunopharmacol. 2002. 2:857–865.
Article16. Lim YJ, Na HS, Yun YS, Choi IS, Oh JS, Rhee JH, Cho BH, Lee HC. Suppressive effects of ginsan on the development of allergic reaction in murine asthmatic model. Int Arch Allergy Immunol. 2009. 150:32–42.
Article17. Na HS, Lim YJ, Yun YS, Choi YH, Oh JS, Rhee JH, Lee HC. Protective effect of Ginsan against Vibrio vulnificus infection. J Bacteriol Virol. 2009. 39:113–118.
Article18. Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995. 155:3292–3295.19. Sozzani S, Luini W, Borsatti A, Polentarutti N, Zhou D, Piemonti L, D'Amico G, Power CA, Wells TN, Gobbi M, Allavena P, Mantovani A. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol. 1997. 159:1993–2000.20. Tanaka A, Hase S, Miyazawa T, Takeuchi K. Up-regulation of cyclooxygenase-2 by inhibition of cyclooxygenase-1: a key to nonsteroidal anti-inflammatory drug-induced intestinal damage. J Pharmacol Exp Ther. 2002. 300:754–761.
Article21. Rimoldi M, Rescigno M. Uptake and presentation of orally administered antigens. Vaccine. 2005. 23:1793–1796.
Article22. Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000. 16:301–332.
Article23. Brandtzaeg P, Baekkevold ES, Farstad IN, Jahnsen FL, Johansen FE, Nilsen EM, Yamanaka T. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol Today. 1999. 20:141–151.
Article24. Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000. 12:495–503.
Article25. Rhoades ER, Cooper AM, Orme IM. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995. 63:3871–3877.
Article26. Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995. 155:3877–3888.27. Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992. 176:1489–1495.
Article28. Zhang Y, Yoneyama H, Wang Y, Ishikawa S, Hashimoto S, Gao JL, Murphy P, Matsushima K. Mobilization of dendritic cell precursors into the circulation by administration of MIP-1alpha in mice. J Natl Cancer Inst. 2004. 96:201–209.
Article29. Lillard JW Jr, Singh UP, Boyaka PN, Singh S, Taub DD, McGhee JR. MIP-1alpha and MIP-1beta differentially mediate mucosal and systemic adaptive immunity. Blood. 2003. 101:807–814.
Article30. Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006. 6:244–251.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stimulatory Effects of Ginsan on the Proliferation and Viability of Mouse Spleen Cells
- Immunomodulatory Activity of Ginsan, a Polysaccharide of Panax Ginseng, on Dendritic Cells
- Perturbation of host responses by Porphyromonas gingivalis biofilm
- Increased humoral antibody response of foot-and-mouth disease virus vaccine in growing pigs pre-treated with poly-γ-glutamic acid
- Humoral response to viral vector COVID-19 vaccine in hemodialysis patients