Anat Cell Biol.
2010 Dec;43(4):317-324. 10.5115/acb.2010.43.4.317.
Lipoic acid suppresses compound 48/80-induced anaphylaxis-like reaction
- Affiliations
-
- 1Department of Anatomy, Chonbuk National University Medical School, Institute for Medical Sciences, Chonbuk National University, Jeonju, Korea. asch@jbnu.ac.kr
- KMID: 1455346
- DOI: http://doi.org/10.5115/acb.2010.43.4.317
Abstract
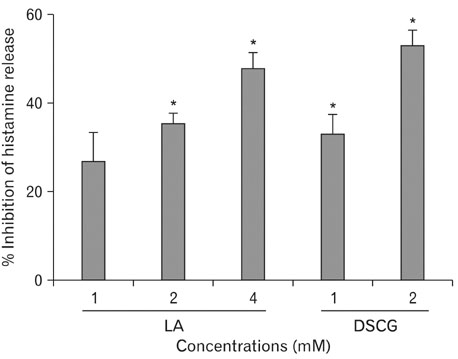
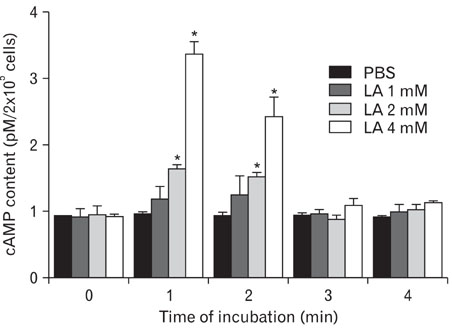
- Alpha-lipoic acid (LA), a naturally occurring dithiol compound, is an essential cofactor in metabolic reactions involved in energy utilization. LA improves glycemic control, reduces diabetic polyneuropathies, atherosclerosis, and allergic inflammation. The effects of LA on mast cell-mediated anaphylactic reactions, however, are unknown. LA dose-dependently inhibited systemic and passive cutaneous anaphylaxis-like reactions in mice induced by compound 48/80, a condensation product of N-methyl-p-methoxyphenethylamine and formaldehyde. Pretreatment with LA, prior to induction of the systemic anaphylaxis-like reaction with compound 48/80, reduced plasma histamine levels in a dose-dependent manner. In our in vitro study, LA decreased histamine release from rat peritoneal mast cells (RPMCs) triggered by compound 48/80. Moreover, an increase in calcium uptake activated by compound 48/80 was inhibited by LA. LA also significantly elevated intracellular cyclic adenosine-3',5' monophosphate (cAMP) levels in RPMCs. This inhibition of mediator release from RPMCs may be due to inhibition of calcium uptake and augmentation of intracellular cAMP levels. Based on these results, we suggest that LA may be a potential remedy for allergy-related diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Akagi M, Katakuse Y, Fukuishi N, Kan T, Akagi R. Superoxide anion-induced histamine release from rat peritoneal mast cells. Biol Pharm Bull. 1994. 17:732–734.2. Bilska A, Włodek L. Lipoic acid - the drug of the future? Pharmacol Rep. 2005. 57:570–577.3. Carvalho RF, Nilsson G, Harvima IT. Increased mast cell expression of PAR-2 in skin inflammatory diseases and release of IL-8 upon PAR-2 activation. Exp Dermatol. 2010. 19:117–122.4. Cho YS, Lee J, Lee TH, et al. alpha-Lipoic acid inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Allergy Clin Immunol. 2004. 114:429–435.5. Choi YH, Yan GH, Chai OH, et al. Inhibition of anaphylaxis-like reaction and mast cell activation by water extract from the fruiting body of Phellinus linteus. Biol Pharm Bull. 2006. 29:1360–1365.6. Fukuishi N, Sakaguchi M, Matsuura S, Nakagawa C, Akagi R, Akagi M. The mechanisms of compound 48/80-induced superoxide generation mediated by A-kinase in rat peritoneal mast cells. Biochem Mol Med. 1997. 61:107–113.7. Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010. 40:1843–1851.8. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008. 454:445–454.9. Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993. 465:359–386.10. Izushi K, Tasaka K. Histamine release from beta-escin-permeabilized rat peritoneal mast cells and its inhibition by intracellular Ca2+ blockers, calmodulin inhibitors and cAMP. Immunopharmacology. 1989. 18:177–186.11. Martynova MG, Bystrova OA, Moiseeva OM, Evdonin AL, Kondratov KA, Medvedeva ND. The presence of ANP in rat peritoneal mast cells. Cell Res. 2005. 15:811–816.12. Nishikawa H, Kitani S. Tea catechins have dual effect on mast cell degranulation induced by compound 48/80. Int Immunopharmacol. 2008. 8:1207–1215.13. Peachell PT, MacGlashan DW Jr, Lichtenstein LM, Schleimer RP. Regulation of human basophil and lung mast cell function by cyclic adenosine monophosphate. J Immunol. 1988. 140:571–579.14. Pearce FL, Ennis M, Truneh A, White JR. Role of intra- and extracellular calcium in histamine release from rat peritoneal mast cells. Agents Actions. 1981. 11:51–54.15. Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009. 1790:1149–1160.16. Shinmei Y, Hossen MA, Okihara K, Sugimoto H, Yamada H, Kamei C. Effect of Brazilian propolis on scratching behavior induced by compound 48/80 and histamine in mice. Int Immunopharmacol. 2004. 4:1431–1436.17. Tasaka K, Mio M, Okamoto M. Intracellular calcium release induced by histamine releasers and its inhibition by some antiallergic drugs. Ann Allergy. 1986. 56:464–469.18. Weston MC, Peachell PT. Regulation of human mast cell and basophil function by cAMP. Gen Pharmacol. 1998. 31:715–719.19. White JR, Ishizaka T, Ishizaka K, Sha'afi R. Direct demonstration of increased intracellular concentration of free calcium as measured by quin-2 in stimulated rat peritoneal mast cell. Proc Natl Acad Sci U S A. 1984. 81:3978–3982.20. Yoshii N, Mio M, Tasaka K. Ca uptake and Ca releasing properties of the endoplasmic reticulum in rat peritoneal mast cells. Immunopharmacology. 1988. 16:107–113.21. Yoshimura T, Hamaguchi E, Usami E, et al. Increased in vitro release of interferon-gamma from ampicillin-stimulated peripheral blood mononuclear cells in Stevens-Johnson syndrome. Biol Pharm Bull. 2004. 27:929–931.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effects of epigallocatechin gallate on compound 48/80-inducedmast cell activation and passive cutaneous anaphylaxis
- Inhibitory Effect of Rubus coreanus on Compound 48/80-induced Anaphylactic Shock and Vascular Permeability in Vivo
- Inhibitory Effect of Corni fructus on Compound 48/80-induced Mast Cell Activation and Vascular Permeability
- Inhibitory Effect of Arctium lappa Linne on Compound 48/80-induced Mast Cell Activation and Vascular Permeability
- Effect of conjugated linoleic acid on compound 48/80-induced pruritus in mice