Anat Cell Biol.
2010 Dec;43(4):310-316. 10.5115/acb.2010.43.4.310.
Amorphigenin inhibits Osteoclast differentiation by suppressing c-Fos and nuclear factor of activated T cells
- Affiliations
-
- 1Department of Orthopedic Surgery, School of Medicine, Wonkwang University, Iksan, Korea.
- 2Department of Anatomy, School of Medicine, Wonkwang University, Iksan, Korea. jjkim@wku.ac.kr
- 3Department of Pathology, School of Medicine, Wonkwang University, Iksan, Korea.
- 4Laboratory of Chemical Genomics, Korea Research Institute of Chemical Technology, Daejeon, Korea.
- KMID: 1455345
- DOI: http://doi.org/10.5115/acb.2010.43.4.310
Abstract
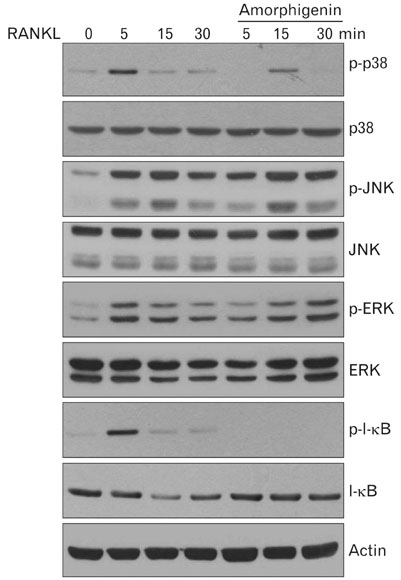
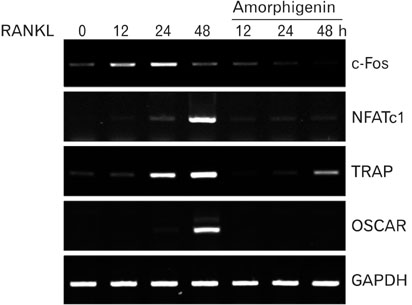
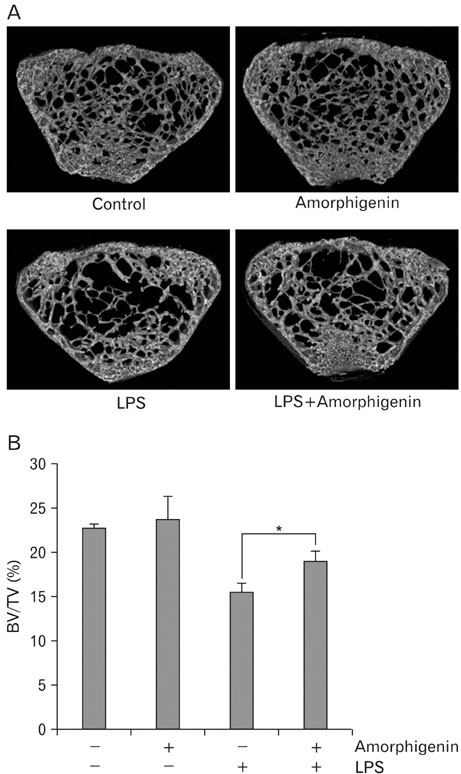
- Among the several rotenoids, amorphigenin is isolated from the leaves of Amopha Fruticosa and it is known that has anti-proliferative effects and anti-cnacer effects in many cell types. The main aim of this study was to investigate the effects of amorphigenin on osteoclast differentiation in vitro and on LPS treated inflammatory bone loss model in vivo. We show here that amorphigenin inhibited RANKL-induced osteoclast differentiation from bone marrow macrophages in a dose dependent manner without cellular toxicity. Anti-osteoclastogenic properties of amorphigenin were based on a down-regulation of c-fos and NFATc1. Amorphigenin markedly inhibited RANKL-induced p38 and NF-kappaB pathways, but other pathways were not affected. Micro-CT analysis of the femurs showed that amorphigenin protected the LPS-induced bone loss. We concluded that amorphigenin can prevent inflammation-induced bone loss. Thus we expect that amorphigenin could be a treatment option for bone erosion caused by inflammation.
Keyword
MeSH Terms
Figure
Reference
-
1. Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. J Periodontol. 2005. 76:11 Suppl. 2066–2074.2. Bharti AC, Takada Y, Aggarwal BB. Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol. 2004. 172:5940–5947.3. Body JJ. Calcitonin for the long-term prevention and treatment of postmenopausal osteoporosis. Bone. 2002. 30:5 Suppl. 75S–79S.4. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003. 423:337–342.5. Everts V, de Vries TJ, Helfrich MH. Osteoclast heterogeneity: lessons from osteopetrosis and inflammatory conditions. Biochim Biophys Acta. 2009. 1792:757–765.6. Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010. 136:1117–1124.7. Hayashi S, Yamane T, Miyamoto A, et al. Commitment and differentiation of stem cells to the osteoclast lineage. Biochem Cell Biol. 1998. 76:911–922.8. Hu JP, Nishishita K, Sakai E, et al. Berberine inhibits RANKL-induced osteoclast formation and survival through suppressing the NF-kappaB and Akt pathways. Eur J Pharmacol. 2008. 580:70–79.9. Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol Endocrinol. 2008. 22:176–185.10. Kloutek E, Popov A, Drenska D, Uzunov P. Experimental research on the hepatoprotective activity of flavonoids isolated from Amorpha fructiosa. Eksp Med Morfol. 1985. 24:50–54.11. Kwak HB, Lee BK, Oh J, et al. Inhibition of osteoclast differentiation and bone resorption by rotenone, through down-regulation of RANKL-induced c-Fos and NFATc1 expression. Bone. 2010. 46:724–731.12. Kwak HB, Yang D, Ha H, et al. Tanshinone IIA inhibits osteoclast differentiation through down-regulation of c-Fos and NFATc1. Exp Mol Med. 2006. 38:256–264.13. Li YJ, Kim TH, Kwak HB, Lee ZH, Lee SY, Jhon GJ. Chloroform extract of deer antler inhibits osteoclast differentiation and bone resorption. J Ethnopharmacol. 2007. 113:191–198.14. Lloyd M. Treatment of postmenopausal osteoporosis. N Engl J Med. 1998. 339:202.15. Miyaura C, Inada M, Matsumoto C, et al. An essential role of cytosolic phospholipase A2alpha in prostaglandin E2-mediated bone resorption associated with inflammation. J Exp Med. 2003. 197:1303–1310.16. O'Regan RM, Gradishar WJ. Selective estrogen-receptor modulators in 2001. Oncology (Williston Park). 2001. 15:1177–1185.17. Park CK, Kim HJ, Kwak HB, et al. Inhibitory effects of Stewartia koreana on osteoclast differentiation and bone resorption. Int Immunopharmacol. 2007. 7:1507–1516.18. Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000. 289:1508–1514.19. Russell RG, Rogers MJ, Frith JC, et al. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J Bone Miner Res. 1999. 14:Suppl 2. 53–65.20. Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med. 2005. 83:170–179.21. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007. 7:292–304.22. Takayanagi H, Iizuka H, Juji T, et al. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000. 43:259–269.23. Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002. 3:889–901.24. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000. 289:1504–1508.25. Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003. 4:638–649.26. Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006. 24:33–63.27. Watts NB. Bisphosphonate treatment of osteoporosis. Clin Geriatr Med. 2003. 19:395–414.28. Zhao Q, Shao J, Chen W, Li YP. Osteoclast differentiation and gene regulation. Front Biosci. 2007. 12:2519–2529.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Iris Koreana NAKAI Inhibits Osteoclast Formation via p38-Mediated Nuclear Factor of Activated T Cells 1 Signaling Pathway
- Effect of Cornus Officinalis on Receptor Activator of Nuclear Factor-kappaB Ligand (RANKL)-induced Osteoclast Differentiation
- Ethyl Acetate Fraction from Cudrania Tricuspidata Inhibits IL-1beta-Stimulated Osteoclast Differentiation through Downregulation of MAPKs, c-Fos and NFATc1
- Methanol Extract of Croton Pycnanthus Benth. Inhibits Osteoclast Differentiation by Suppressing the MAPK and NF-kappaB Signaling Pathways
- Aster saponin A 2 inhibits osteoclastogenesis through mitogenactivated protein kinase-c-Fos-NFATc1 signaling pathway