J Bone Metab.
2014 Nov;21(4):269-275. 10.11005/jbm.2014.21.4.269.
Methanol Extract of Croton Pycnanthus Benth. Inhibits Osteoclast Differentiation by Suppressing the MAPK and NF-kappaB Signaling Pathways
- Affiliations
-
- 1Department of Cell and Developmental Biology, BK21 Program and Dental Research Institute, Seoul National University, Seoul, Korea. hhbkim@snu.ac.kr
- KMID: 2170055
- DOI: http://doi.org/10.11005/jbm.2014.21.4.269
Abstract
- BACKGROUND
Osteoclasts are differentiated from monocytes/macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-kappa B (NF-kappaB) ligand (RANKL). Croton pycnanthus Benth. (CPB) is a herbal plant that belongs to Euphorbiaceae family. The aim of this study was to investigate the effects of CPB on osteoclastogenesis and RANKL-dependent signaling pathways.
METHODS
Methanol extract of CPB was obtained from International Biological Material Research Center. Osteoclast differentiation was achieved by culturing mouse bone marrow-derived macrophages (BMMs) with M-CSF and RANKL. Osteoclast numbers were evaluated by counting multinuclear cells positive for tartrate-resistant acid phosphatase (TRAP). mRNA and protein levels were analyzed by real-time polymerase chain reaction (PCR) and Western blotting, respectively. The activation of signaling molecules were assessed after acute stimulation of cells with high dose of RANKL by Western blotting with phospho-specific antibodies.
RESULTS
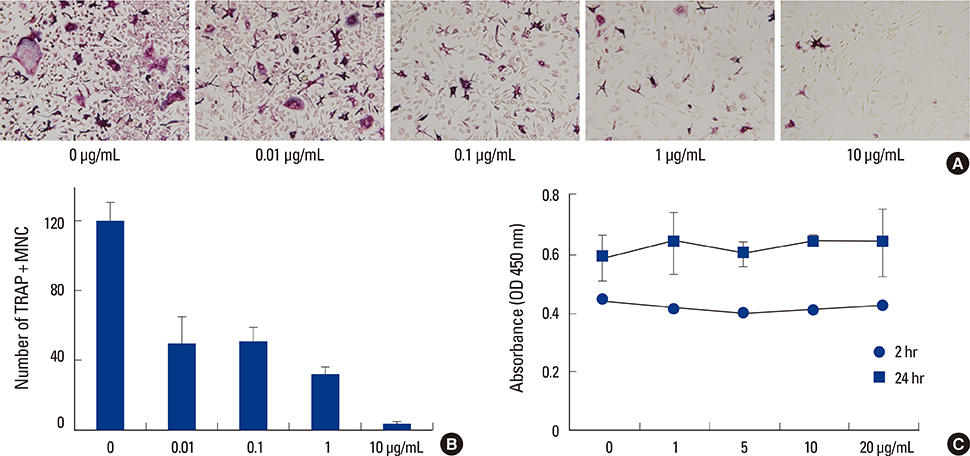
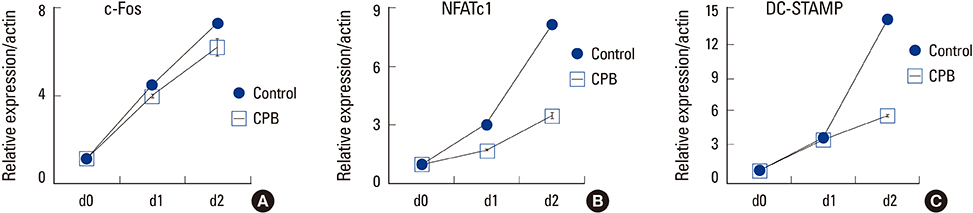
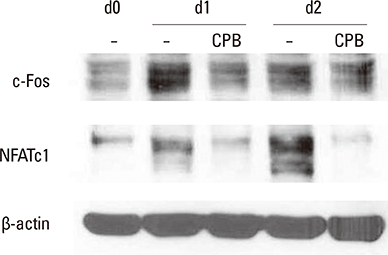
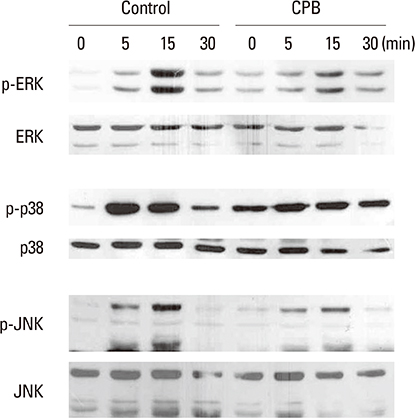
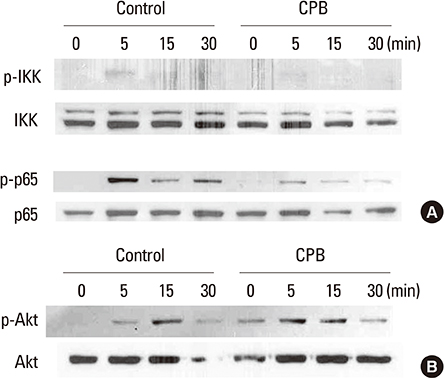
CPB reduced the generation of TRAP-positive multinucleated cells and the activation of mitogen-activated protein kinase (MAPK) and NF-kappaB signaling pathways. The induction of the expression of c-Fos, nuclear factor-activated T cells c1 (NFATc1) and dendritic cell-specific transmembrane protein (DC-STAMP) by RANKL was also suppressed.
CONCLUSIONS
CPB exerts negative effects on osteoclast differentiation in response to the RANKL. The inhibitory mechanism involves the suppression of MAPK and NF-kappaB signaling pathways and subsequently the down-regulation of c-Fos and NFATc1 transcription factors.
Keyword
MeSH Terms
-
Acid Phosphatase
Animals
Antibodies, Phospho-Specific
Blotting, Western
Cell Differentiation
Colony-Stimulating Factors
Croton*
Down-Regulation
Euphorbiaceae
Humans
Macrophage Colony-Stimulating Factor
Macrophages
Methanol*
Mice
NF-kappa B*
NFATC Transcription Factors
Osteoclasts*
Plants
Protein Kinases
RANK Ligand
Real-Time Polymerase Chain Reaction
Receptor Activator of Nuclear Factor-kappa B
RNA, Messenger
T-Lymphocytes
Acid Phosphatase
Antibodies, Phospho-Specific
Colony-Stimulating Factors
Macrophage Colony-Stimulating Factor
Methanol
NF-kappa B
NFATC Transcription Factors
Protein Kinases
RANK Ligand
RNA, Messenger
Receptor Activator of Nuclear Factor-kappa B
Figure
Reference
-
1. Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998; 93:165–176.
Article2. Fuller K, Wong B, Fox S, et al. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998; 188:997–1001.
Article3. Miyamoto T, Ohneda O, Arai F, et al. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood. 2001; 98:2544–2554.
Article4. Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901.
Article5. Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev. 2005; 208:126–140.
Article6. Aliprantis AO, Ueki Y, Sulyanto R, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008; 118:3775–3789.
Article7. Asagiri M, Sato K, Usami T, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005; 202:1261–1269.
Article8. Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem Biophys Res Commun. 2003; 305:211–214.
Article9. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007; 7:292–304.
Article10. Yagi M, Miyamoto T, Sawatani Y, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005; 202:345–351.
Article11. Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999; 13:2412–2424.
Article12. Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999; 397:315–323.
Article13. Jimi E, Akiyama S, Tsurukai T, et al. Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol. 1999; 163:434–442.14. Lee SE, Woo KM, Kim SY, et al. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 2002; 30:71–77.
Article15. Iotsova V, Caamaño J, Loy J, et al. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997; 3:1285–1289.16. Wei S, Teitelbaum SL, Wang MW, et al. Receptor activator of nuclear factor-kappa b ligand activates nuclear factor-kappa b in osteoclast precursors. Endocrinology. 2001; 142:1290–1295.
Article17. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999; 13:2905–2927.
Article18. Zhang C, Dou CE, Xu J, et al. DC-STAMP, the key fusion-mediating molecule in osteoclastogenesis. J Cell Physiol. 2014; 229:1330–1335.
Article19. Roodman GD, Windle JJ. Paget disease of bone. J Clin Invest. 2005; 115:200–208.
Article20. Riba J, Valle M, Urbano G, et al. Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther. 2003; 306:73–83.
Article21. Del Valle Mendoza J, Pumarola T, Gonzales LA, et al. Antiviral activity of maca (Lepidium meyenii) against human influenza virus. Asian Pac J Trop Med. 2014; 7S1:S415–S420.
Article22. Arora RB, Kapoor V, Basu N, et al. Anti-inflammatory studies on Curcuma longa (turmeric). Indian J Med Res. 1971; 59:1289–1295.23. Manhas A, Khanna V, Prakash P, et al. Curcuma oil reduces endothelial cell-mediated inflammation in postmyocardial ischemia/reperfusion in rats. J Cardiovasc Pharmacol. 2014; 64:228–236.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NF-kappaB Activation in T Helper 17 Cell Differentiation
- Signaling Pathways in Osteoclast Differentiation
- Differential Regulation of NF-kappaB Signaling during Human Cytomegalovirus Infection
- NF-kappaB-Mediated Regulation of Osteoclastogenesis
- Simvastatin inhibits osteoclast differentiation by scavenging reactive oxygen species