Anat Cell Biol.
2011 Sep;44(3):226-237. 10.5115/acb.2011.44.3.226.
Co-localization of activating transcription factor 3 and phosphorylated c-Jun in axotomized facial motoneurons
- Affiliations
-
- 1Department of Anatomy, Yonsei University Wonju College of Medicine, Wonju, Korea. bpcho@yonsei.ac.kr
- 2Department of Anatomy and Neuroscience, Eulji University School of Medicine, Daejeon, Korea.
- 3Department of Physiology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 1447435
- DOI: http://doi.org/10.5115/acb.2011.44.3.226
Abstract
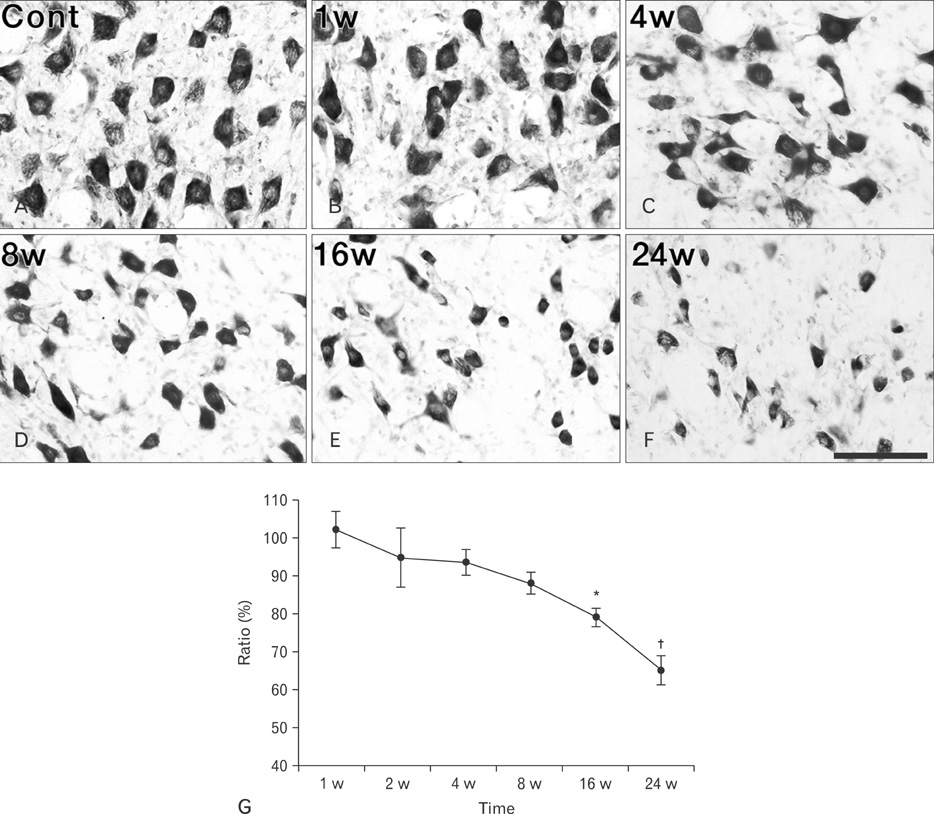
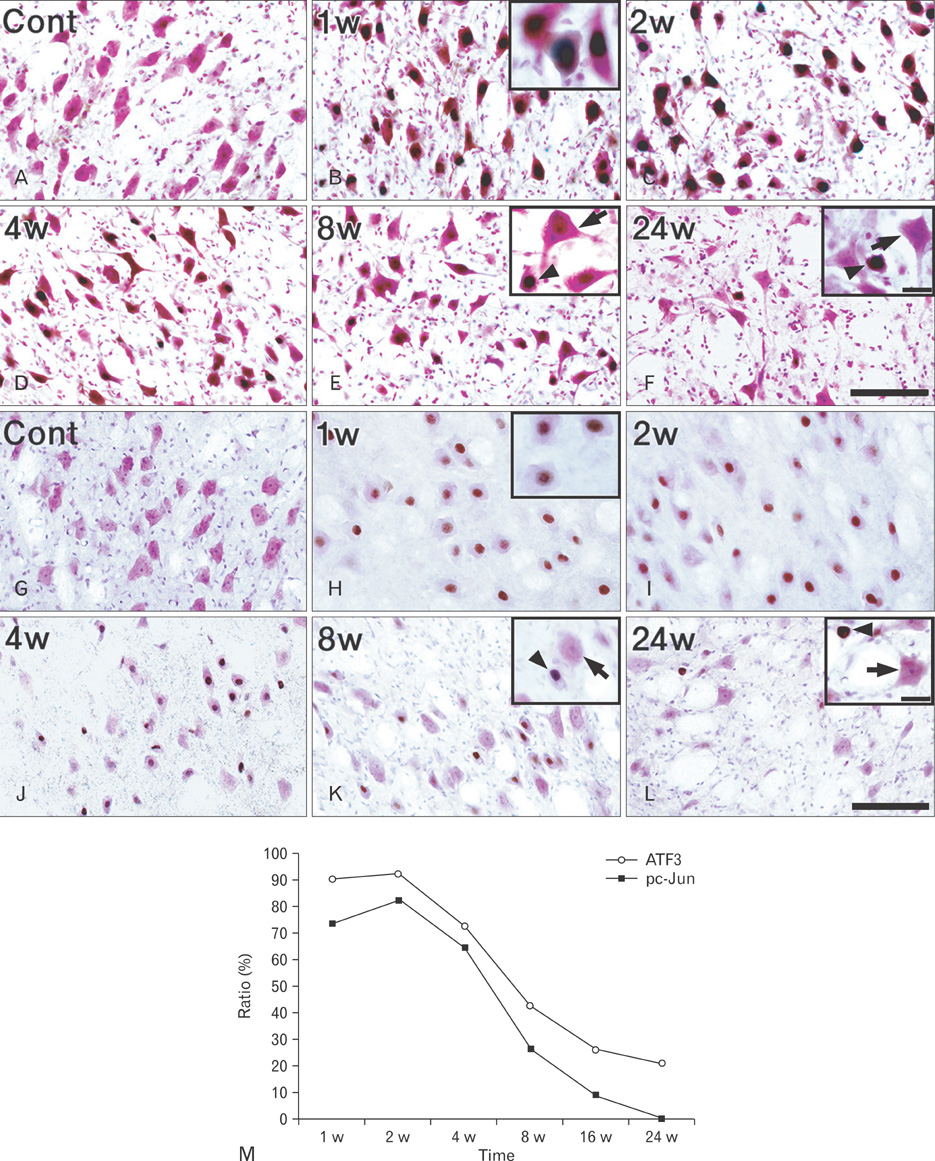
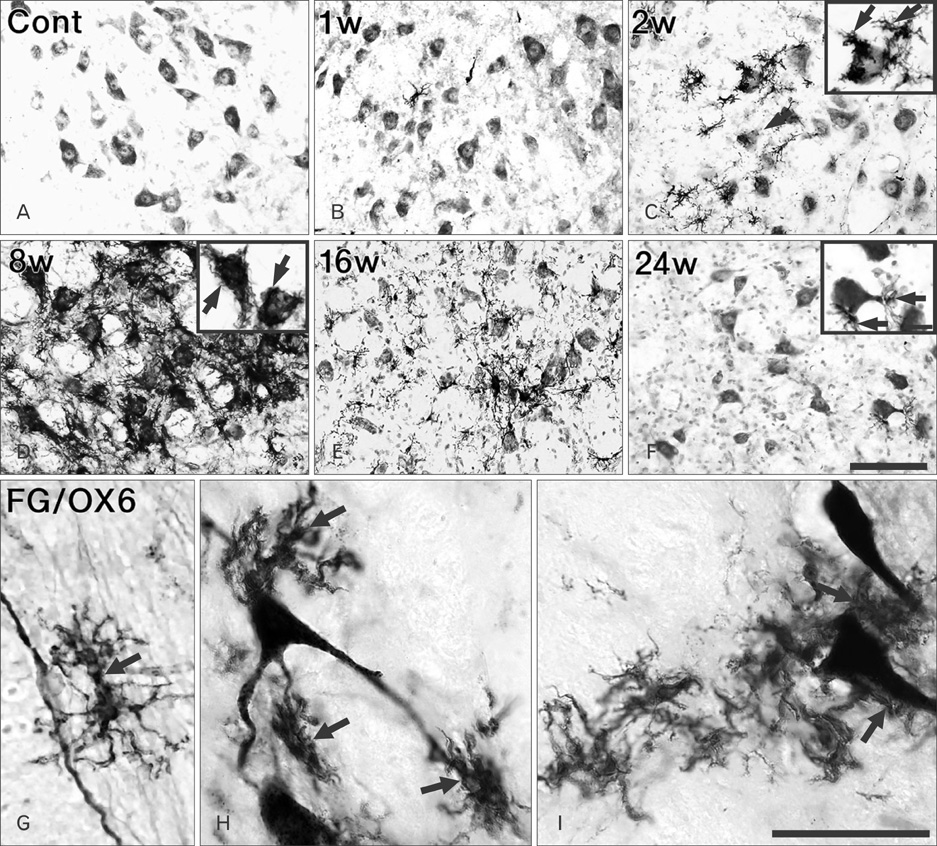
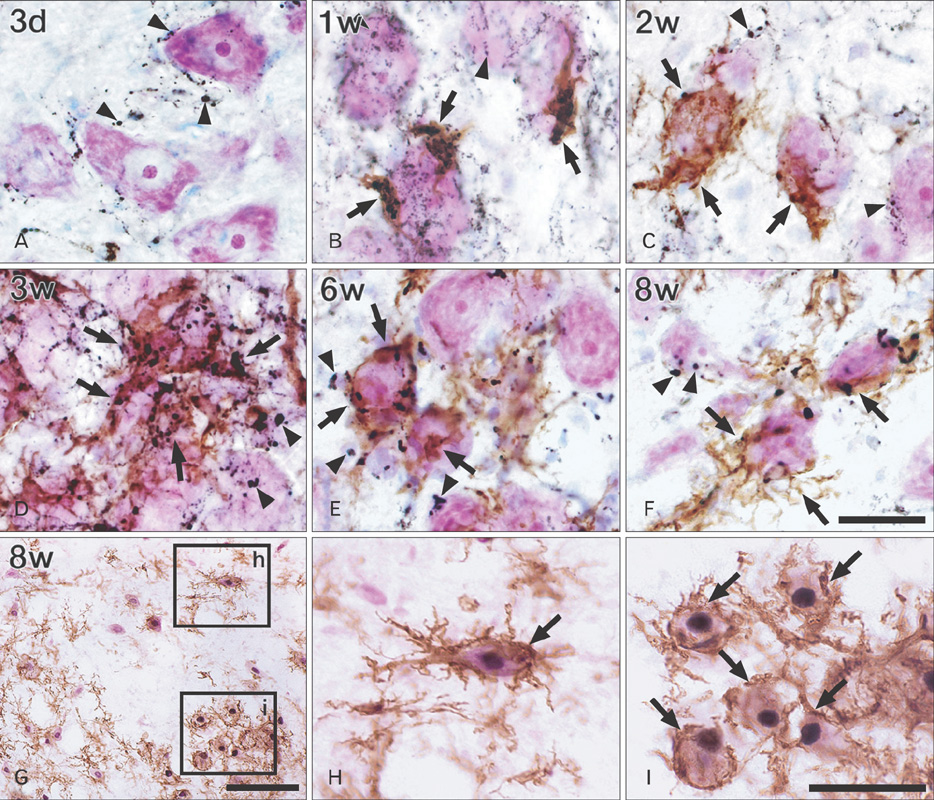
- Activating transcription factor 3 (ATF3) and c-Jun play key roles in either cell death or cell survival, depending on the cellular background. To evaluate the functional significance of ATF3/c-Jun in the peripheral nervous system, we examined neuronal cell death, activation of ATF3/c-Jun, and microglial responses in facial motor nuclei up to 24 weeks after an extracranial facial nerve axotomy in adult rats. Following the axotomy, neuronal survival rate was progressively but significantly reduced to 79.1% at 16 weeks post-lesion (wpl) and to 65.2% at 24 wpl. ATF3 and phosphorylated c-Jun (pc-Jun) were detected in the majority of ipsilateral facial motoneurons with normal size and morphology during the early stage of degeneration (1-2 wpl). Thereafter, the number of facial motoneurons decreased gradually, and both ATF3 and pc-Jun were identified in degenerating neurons only. ATF3 and pc-Jun were co-localized in most cases. Additionally, a large number of activated microglia, recognized by OX6 (rat MHC II marker) and ED1 (phagocytic marker), gathered in the ipsilateral facial motor nuclei. Importantly, numerous OX6- and ED1-positive, phagocytic microglia closely surrounded and ingested pc-Jun-positive, degenerating neurons. Taken together, our results indicate that long-lasting co-localization of ATF3 and pc-Jun in axotomized facial motoneurons may be related to degenerative cascades provoked by an extracranial facial nerve axotomy.
Keyword
MeSH Terms
Figure
Reference
-
1. Haas CA, Donath C, Kreutzberg GW. Differential expression of immediate early genes after transection of the facial nerve. Neuroscience. 1993. 53:91–99.2. Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990. 4:477–485.3. Herdegen T, Skene P, Bähr M. The c-Jun transcription factor: bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997. 20:227–231.4. Song DY, Yang YC, Shin DH, Sugama S, Kim YS, Lee BH, Joh TH, Cho BP. Axotomy-induced dopaminergic neurodegeneration is accompanied with c-Jun phosphorylation and activation transcription factor 3 expression. Exp Neurol. 2008. 209:268–278.5. Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000. 15:170–182.6. Chen BP, Wolfgang CD, Hai T. Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol. 1996. 16:1157–1168.7. Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999. 7:321–335.8. Kawauchi J, Zhang C, Nobori K, Hashimoto Y, Adachi MT, Noda A, Sunamori M, Kitajima S. Transcriptional repressor activating transcription factor 3 protects human umbilical vein endothelial cells from tumor necrosis factor-alpha-induced apoptosis through down-regulation of p53 transcription. J Biol Chem. 2002. 277:39025–39034.9. Nakagomi S, Suzuki Y, Namikawa K, Kiryu-Seo S, Kiyama H. Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. J Neurosci. 2003. 23:5187–5196.10. Hartman MG, Lu D, Kim ML, Kociba GJ, Shukri T, Buteau J, Wang X, Frankel WL, Guttridge D, Prentki M, Grey ST, Ron D, Hai T. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol. 2004. 24:5721–5732.11. Mashima T, Udagawa S, Tsuruo T. Involvement of transcriptional repressor ATF3 in acceleration of caspase protease activation during DNA damaging agent-induced apoptosis. J Cell Physiol. 2001. 188:352–358.12. Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991. 88:3720–3724.13. Leah JD, Herdegen T, Bravo R. Selective expression of Jun proteins following axotomy and axonal transport block in peripheral nerves in the rat: evidence for a role in the regeneration process. Brain Res. 1991. 566:198–207.14. Sommer C, Gass P, Kiessling M. Selective c-JUN expression in CA1 neurons of the gerbil hippocampus during and after acquisition of an ischemia-tolerant state. Brain Pathol. 1995. 5:135–144.15. Dragunow M, Beilharz E, Sirimanne E, Lawlor P, Williams C, Bravo R, Gluckman P. Immediate-early gene protein expression in neurons undergoing delayed death, but not necrosis, following hypoxic-ischaemic injury to the young rat brain. Brain Res Mol Brain Res. 1994. 25:19–33.16. Dragunow M, Young D, Hughes P, MacGibbon G, Lawlor P, Singleton K, Sirimanne E, Beilharz E, Gluckman P. Is c-Jun involved in nerve cell death following status epilepticus and hypoxic-ischaemic brain injury? Brain Res Mol Brain Res. 1993. 18:347–352.17. Ferrer I, Olive M, Ribera J, Planas AM. Naturally occurring (programmed) and radiation-induced apoptosis are associated with selective c-Jun expression in the developing rat brain. Eur J Neurosci. 1996. 8:1286–1298.18. Oo TF, Henchcliffe C, James D, Burke RE. Expression of c-fos, c-jun, and c-jun N-terminal kinase (JNK) in a developmental model of induced apoptotic death in neurons of the substantia nigra. J Neurochem. 1999. 72:557–564.19. Crocker SJ, Lamba WR, Smith PD, Callaghan SM, Slack RS, Anisman H, Park DS. c-Jun mediates axotomy-induced dopamine neuron death in vivo. Proc Natl Acad Sci U S A. 2001. 98:13385–13390.20. Anderson AJ, Cummings BJ, Cotman CW. Increased immunoreactivity for Jun- and Fos-related proteins in Alzheimer's disease: association with pathology. Exp Neurol. 1994. 125:286–295.21. Martín G, Seguí J, Díaz-Villoslada P, Montalbán X, Planas AM, Ferrer I. Jun expression is found in neurons located in the vicinity of subacute plaques in patients with multiple sclerosis. Neurosci Lett. 1996. 212:95–98.22. Cho BP, Song DY, Sugama S, Shin DH, Shimizu Y, Kim SS, Kim YS, Joh TH. Pathological dynamics of activated microglia following medial forebrain bundle transection. Glia. 2006. 53:92–102.23. Cho BP, Sugama S, Shin DH, DeGiorgio LA, Kim SS, Kim YS, Lim SY, Park KC, Volpe BT, Cho S, Joh TH. Microglial phagocytosis of dopamine neurons at early phases of apoptosis. Cell Mol Neurobiol. 2003. 23:551–560.24. Sugama S, Cho BP, Degiorgio LA, Shimizu Y, Kim SS, Kim YS, Shin DH, Volpe BT, Reis DJ, Cho S, Joh TH. Temporal and sequential analysis of microglia in the substantia nigra following medial forebrain bundle axotomy in rat. Neuroscience. 2003. 116:925–933.25. Takeda M, Kato H, Takamiya A, Yoshida A, Kiyama H. Injury-specific expression of activating transcription factor-3 in retinal ganglion cells and its colocalized expression with phosphorylated c-Jun. Invest Ophthalmol Vis Sci. 2000. 41:2412–2421.26. Guntinas-Lichius O, Neiss WF, Gunkel A, Stennert E. Differences in glial, synaptic and motoneuron responses in the facial nucleus of the rat brainstem following facial nerve resection and nerve suture reanastomosis. Eur Arch Otorhinolaryngol. 1994. 251:410–417.27. Johnson IP, Duberley RM. Motoneuron survival and expression of neuropeptides and neurotrophic factor receptors following axotomy in adult and ageing rats. Neuroscience. 1998. 84:141–150.28. Mattsson P, Meijer B, Svensson M. Extensive neuronal cell death following intracranial transection of the facial nerve in the adult rat. Brain Res Bull. 1999. 49:333–341.29. Gehrmann J, Mies G, Bonnekoh P, Banati R, Iijima T, Kreutzberg GW, Hossmann KA. Microglial reaction in the rat cerebral cortex induced by cortical spreading depression. Brain Pathol. 1993. 3:11–17.30. Graeber MB, López-Redondo F, Ikoma E, Ishikawa M, Imai Y, Nakajima K, Kreutzberg GW, Kohsaka S. The microglia/macrophage response in the neonatal rat facial nucleus following axotomy. Brain Res. 1998. 813:241–253.31. Graeber MB, Streit WJ, Kreutzberg GW. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J Neurosci Res. 1988. 21:18–24.32. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1998. 4th ed. San Diego: Academic Press.33. Damoiseaux JG, Döpp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology. 1994. 83:140–147.34. Streit WJ, Kreutzberg GW. Response of endogenous glial cells to motor neuron degeneration induced by toxic ricin. J Comp Neurol. 1988. 268:248–263.35. Dragunow M, Preston K. The role of inducible transcription factors in apoptotic nerve cell death. Brain Res Brain Res Rev. 1995. 21:1–28.36. Barron KD. The axotomy response. J Neurol Sci. 2004. 220:119–121.37. Lindwall C, Kanje M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol Cell Neurosci. 2005. 29:269–282.38. Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, Lipp HP, Aguzzi A, Wagner EF, Behrens A. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004. 43:57–67.39. Brecht S, Kirchhof R, Chromik A, Willesen M, Nicolaus T, Raivich G, Wessig J, Waetzig V, Goetz M, Claussen M, Pearse D, Kuan CY, Vaudano E, Behrens A, Wagner E, Flavell RA, Davis RJ, Herdegen T. Specific pathophysiological functions of JNK isoforms in the brain. Eur J Neurosci. 2005. 21:363–377.40. Pittman RN, Messam CA, Mills JC. Koliatsos VE, Ratan RR, editors. Asynchronous death as a characteristics feature of apoptosis. Cell Death and Diseases of the Nervous System. 1998. Totowa: Hamana Press;29–44.41. Reimold AM, Grusby MJ, Kosaras B, Fries JW, Mori R, Maniwa S, Clauss IM, Collins T, Sidman RL, Glimcher MJ, Glimcher LH. Chondrodysplasia and neurological abnormalities in ATF-2-deficient mice. Nature. 1996. 379:262–265.42. Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997. 9:240–246.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Expression of Activating Transcription Factor-2 and c-Jun in the Immature and Adult Rat Hippocampus Following Lithium-Pilocarpine Induced Status Epilepticus
- Human Leptin Protein Induces Proliferation of A549 Cells via Inhibition of PKR-Like ER Kinase and Activating Transcription Factor-6 Mediated Apoptosis
- Kahweol from Coffee Induces Apoptosis by Upregulating Activating Transcription Factor 3 in Human Colorectal Cancer Cells
- Regulation of Cartilage Development and Diseases by Transcription Factors
- Is Activating Transcription Factor 3 Up-Regulated in Patients with Hypospadias?